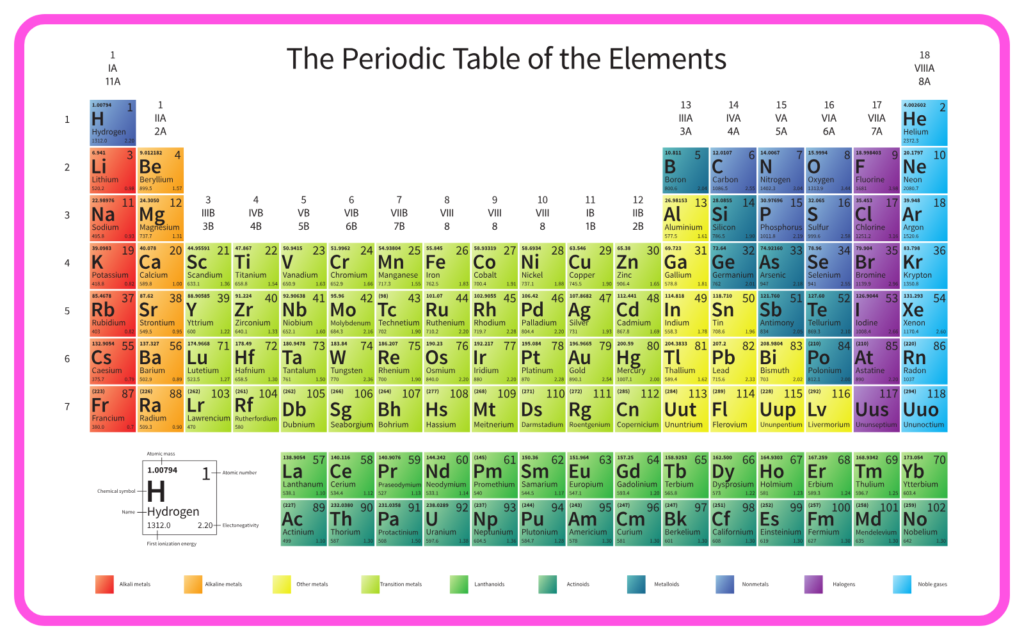
Making Order Out Of Chaos-The Modern Periodic Table
Key Notes:
Introduction to the Periodic Table
- The periodic table organizes elements based on their properties and atomic structure.
- Initially chaotic, the modern periodic table brought order by arranging elements systematically.
Historical Development
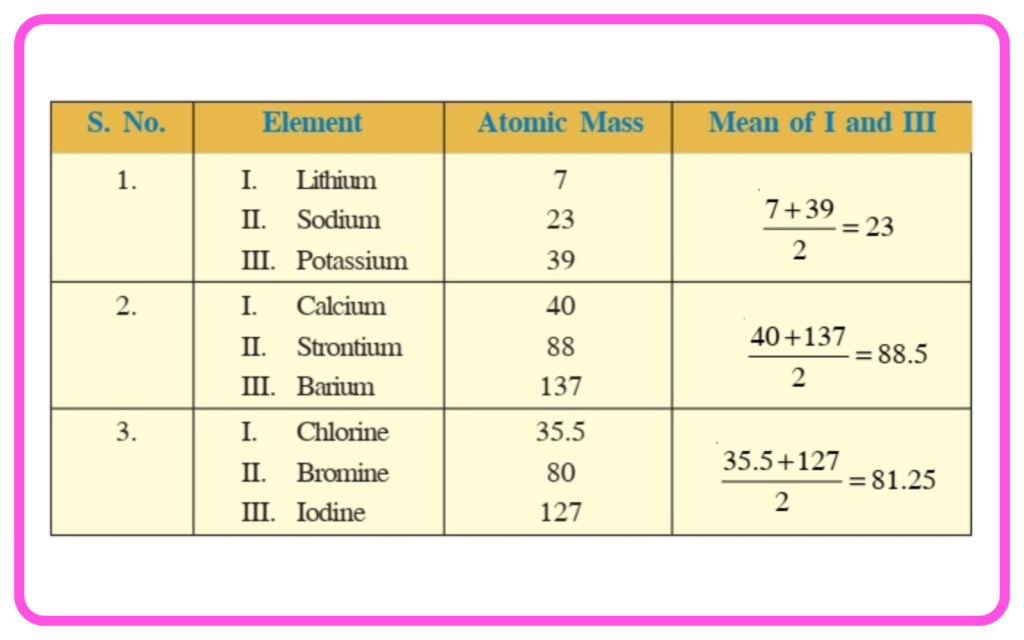
- Dobereiner’s Triads: Grouped elements into triads with similar properties (e.g., Li, Na, K).
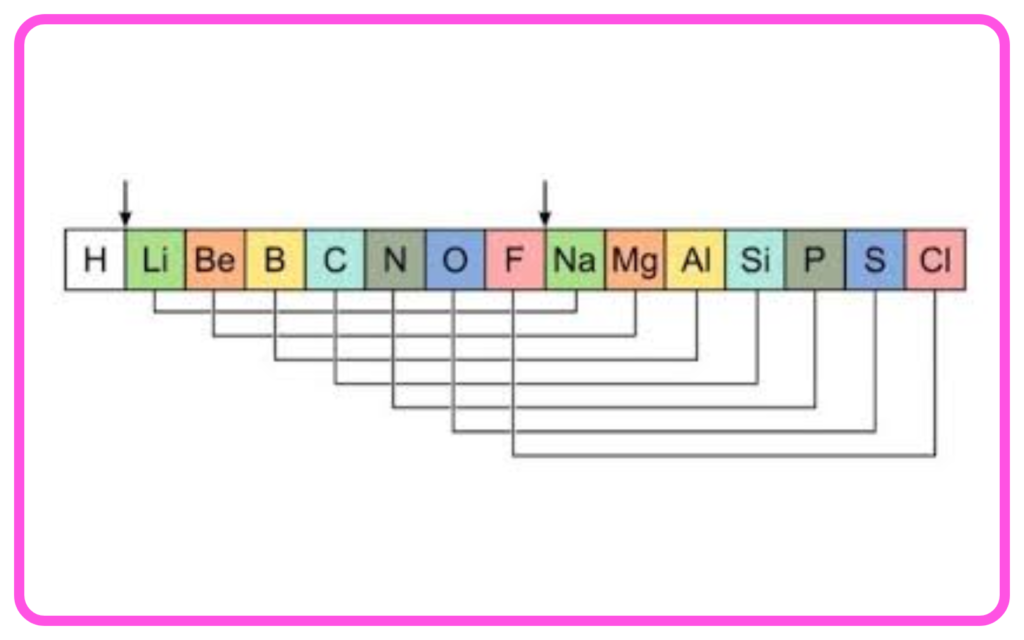
- Newlands’ Law of Octaves: Noted that every eighth element had similar properties.
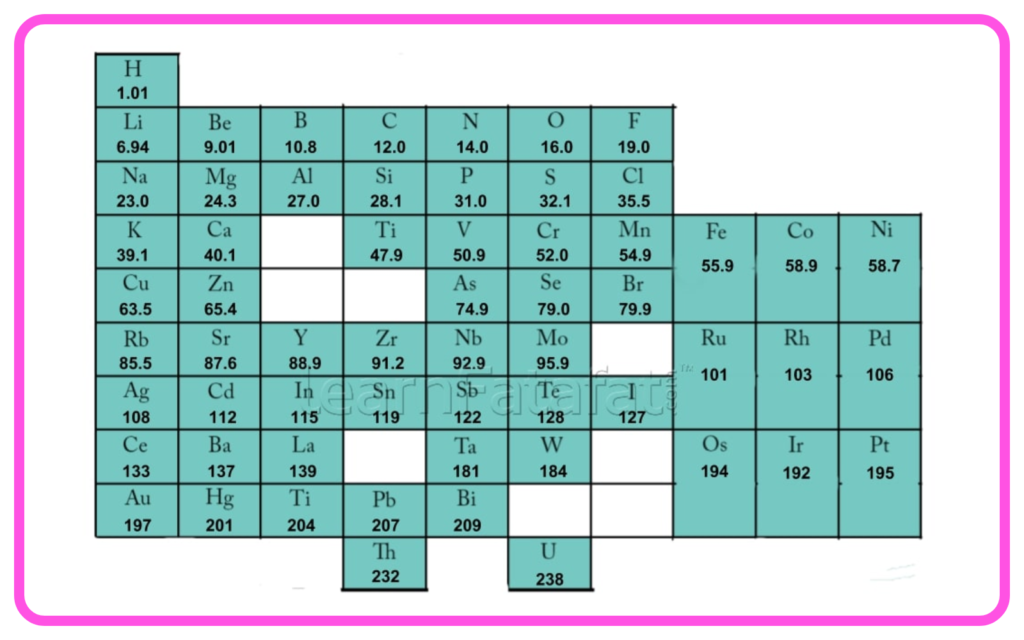
- Mendeleev’s Periodic Table: Organized elements by increasing atomic mass, leaving gaps for undiscovered elements.
- Moseley’s Contribution: Rearranged elements by increasing atomic number, resolving inconsistencies in Mendeleev’s table.
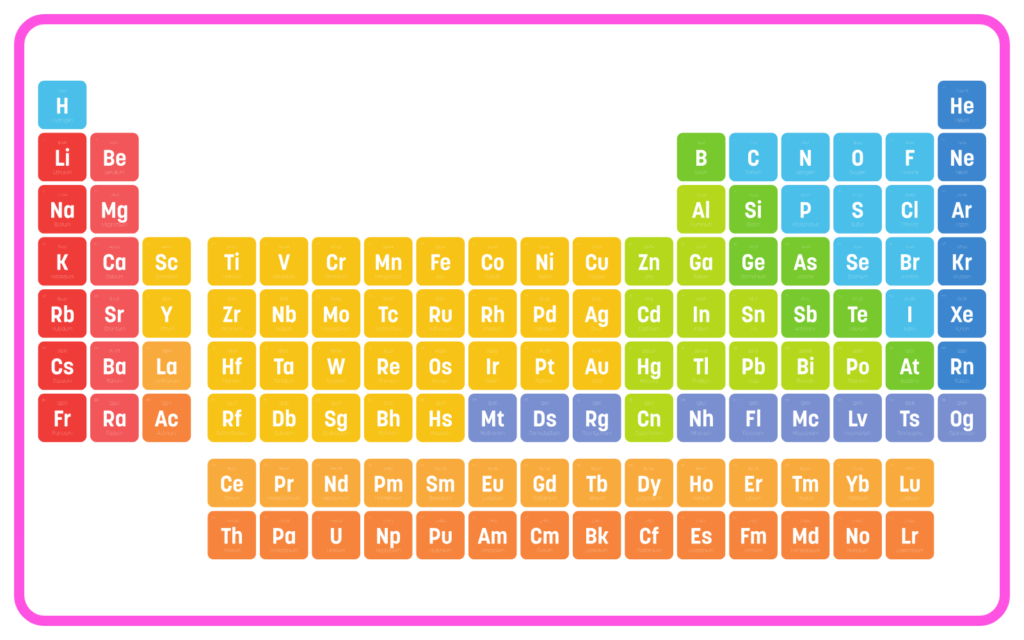
Structure of the Modern Periodic Table
- Elements are arranged in increasing atomic number.
- Periods: Horizontal rows (7 in total), indicating the number of energy shells.
- Groups: Vertical columns (18 in total), with elements having similar valence electron configurations.
Periodic Trends
- Atomic Size: Decreases across a period (due to increased nuclear charge) and increases down a group (due to additional energy levels).
- Ionization Energy: Increases across a period and decreases down a group.
- Electronegativity: Increases across a period and decreases down a group.
- Metallic Character: Decreases across a period and increases down a group.
Classification of Elements
- Metals: Found on the left and center; good conductors of heat and electricity.
- Non-Metals: Found on the right; poor conductors.
- Metalloids: Border metals and non-metals, exhibiting mixed properties.

Significance of the Modern Periodic Table
- Predicts properties of elements based on their position.
- Aids in understanding chemical reactions and bonding.
- Organizes elements for practical applications in science and technology.
Key Features
- Group numbers correlate with the number of valence electrons (for Groups 1-2 and 13-18).
- Period numbers indicate the highest energy level of electrons in an atom.
- Noble gases (Group 18) are inert due to a full valence shell.
Current Use
- Expands to include newly discovered elements (e.g., superheavy elements).
- Facilitates research in chemistry, physics, and material science.
Let’s practice!

