Making Order Out Of Chaos-Mendeleev’s Periodic Table
Key Notes:
Historical Background:
- Chemistry in the mid-19th century was disorganized, with about 63 known elements.
- Scientists sought a system to classify elements based on their properties.
Dmitri Mendeleev:
- A Russian chemist who introduced the first periodic table in 1869.

- His periodic table arranged elements in increasing order of atomic masses.
Mendeleev’s Approach:
- Grouped elements with similar chemical properties into vertical columns (groups).
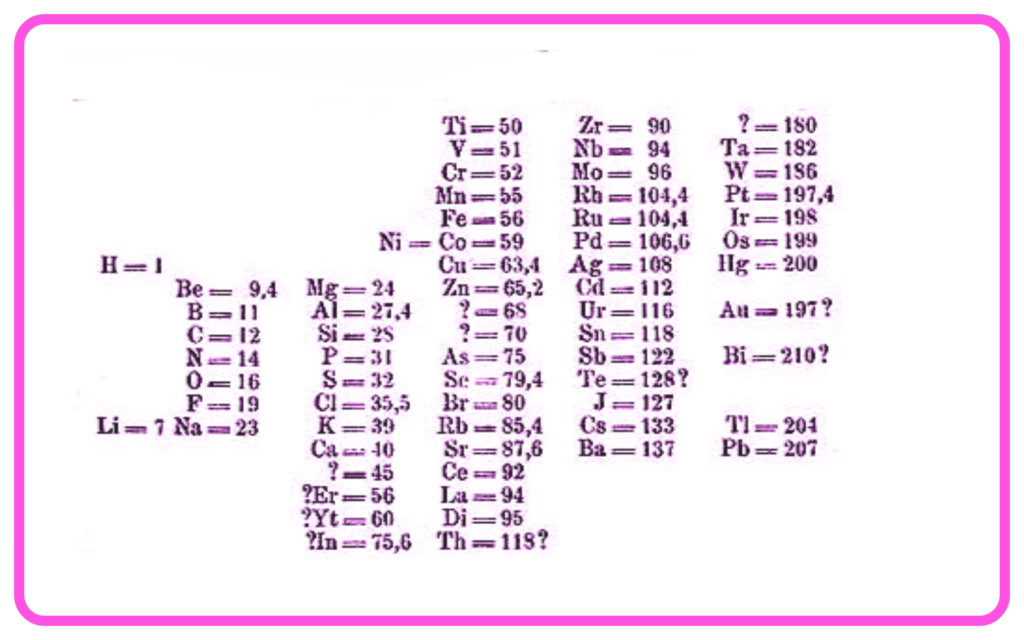
- Left gaps for undiscovered elements and predicted their properties accurately.
Periodic Law:
- Mendeleev formulated the Periodic Law: “The properties of elements are a periodic function of their atomic masses.”
- Elements showed recurring (“periodic”) properties at regular intervals.
Key Features of Mendeleev’s Table:
- Vertical Groups: Contained elements with similar properties.
- Horizontal Periods: Represented a progression in properties.
- Gaps for Undiscovered Elements: For example, he left spaces for scandium, gallium, and germanium.
Predictions and Discoveries:
- Mendeleev predicted properties of elements like eka-aluminum (gallium) and eka-silicon (germanium).


- Later discoveries matched his predictions, proving the reliability of his table.
Challenges in Mendeleev’s Table:
- Certain elements did not fit perfectly when arranged by atomic mass (e.g., iodine and tellurium).
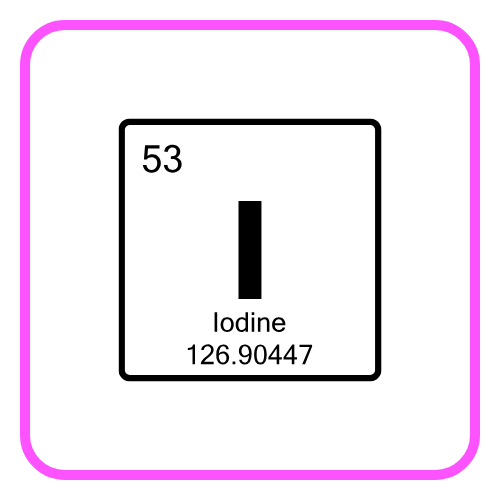

- Isotopes and atomic numbers were not known at the time.
Significance of Mendeleev’s Work:
- Brought order and systematic study to chemistry.
- Laid the foundation for the modern periodic table.
Limitations:
- Could not explain the reasons for periodicity.
- Some anomalies were later resolved by the discovery of atomic number and isotopes.
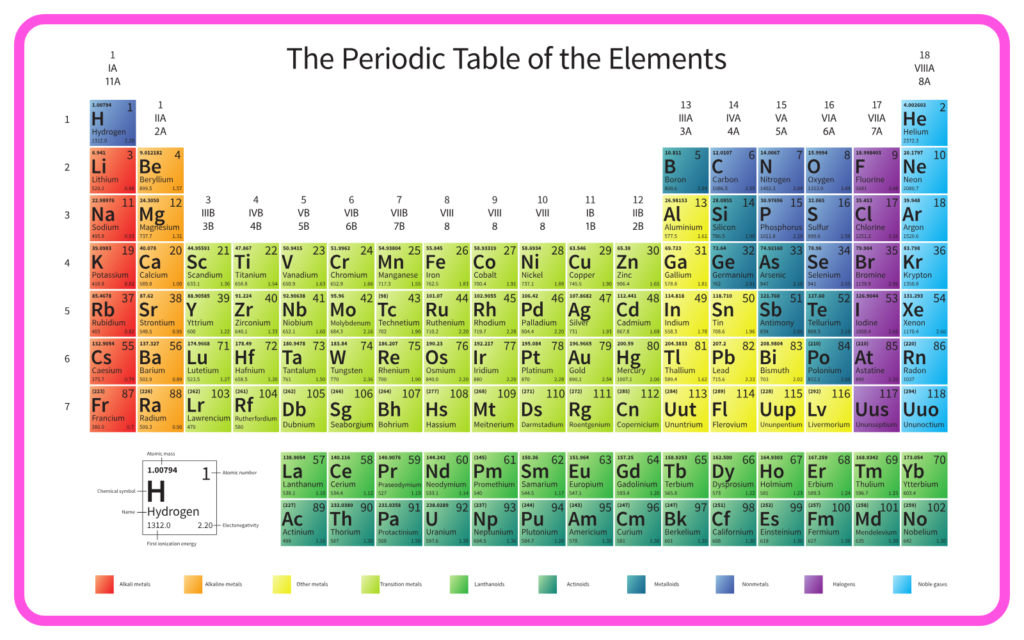
Modern Periodic Table:
- Based on atomic number (introduced by Henry Moseley in 1913).
- Improved upon Mendeleev’s model but retained the fundamental periodic law.
Impact:
- Mendeleev’s table remains a cornerstone in the study of chemistry.
- It demonstrated the power of scientific prediction and organization.
Let’s practice!

