Chemical Properties Of Carbon Compounds
Key Notes:
Combustion:
- Carbon compounds, like hydrocarbons, burn in the presence of oxygen to produce carbon dioxide, water, heat, and light.

- Complete Combustion: Occurs with sufficient oxygen supply, producing carbon dioxide and water.
- Example: Methane CH4 + 2O2 → CO2 + 2H2O → CO2 + 2H2O
- Incomplete Combustion: Limited oxygen supply results in carbon monoxide or soot.
- Example: Ethane in limited oxygen produces carbon monoxide.
Oxidation:
- Carbon compounds can undergo oxidation reactions to form alcohols, aldehydes, acids, etc.
- Oxidizing agents like alkaline potassium permanganate (KMnO₄) or acidic potassium dichromate (K₂Cr₂O₇) can oxidize ethanol to ethanoic acid.

Addition Reactions (in Unsaturated Hydrocarbons):
- Occurs with alkenes and alkynes due to the presence of double or triple bonds.
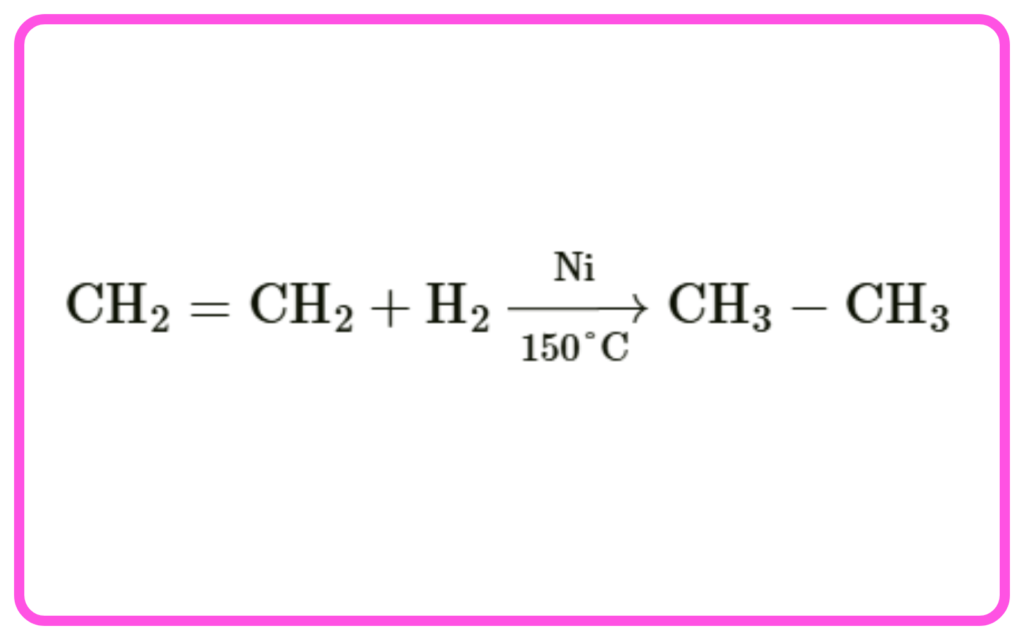
- Hydrogenation: Addition of hydrogen to unsaturated hydrocarbons to form saturated ones using a nickel or platinum catalyst.
- Example: Ethene + Hydrogen → Ethane.
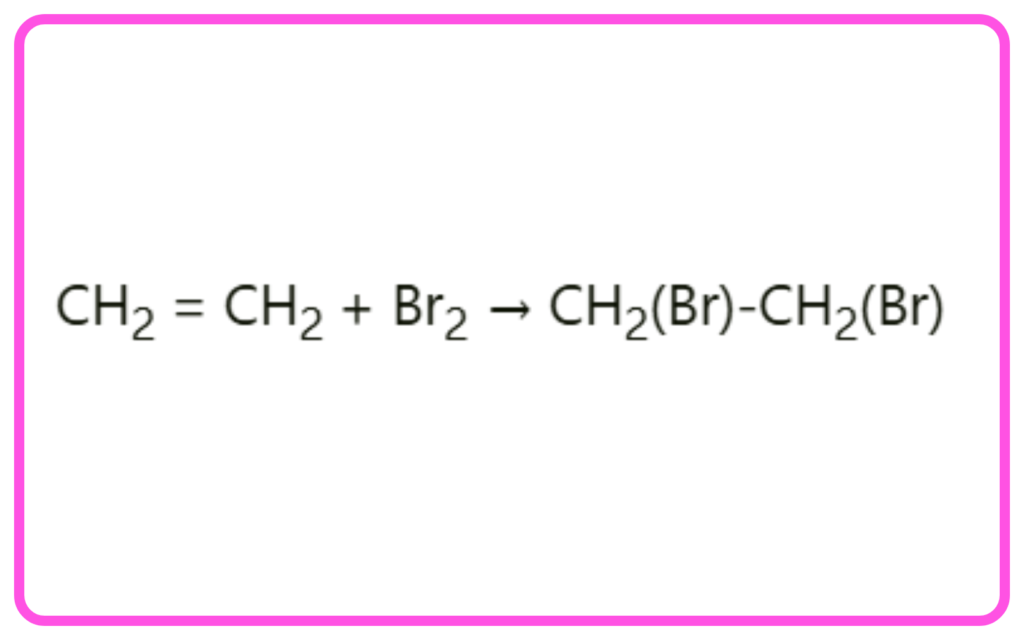
- Halogenation: Addition of halogens like chlorine or bromine.
- Example: Ethene + Bromine → 1,2-Dibromoethane.
Substitution Reactions (in Saturated Hydrocarbons):
- Involves replacing one or more hydrogen atoms in an alkane with another atom (e.g., halogen).
- These reactions are common in alkanes due to their stability.
- Example: Methane + Chlorine (in the presence of sunlight) → Chloromethane + Hydrogen Chloride.
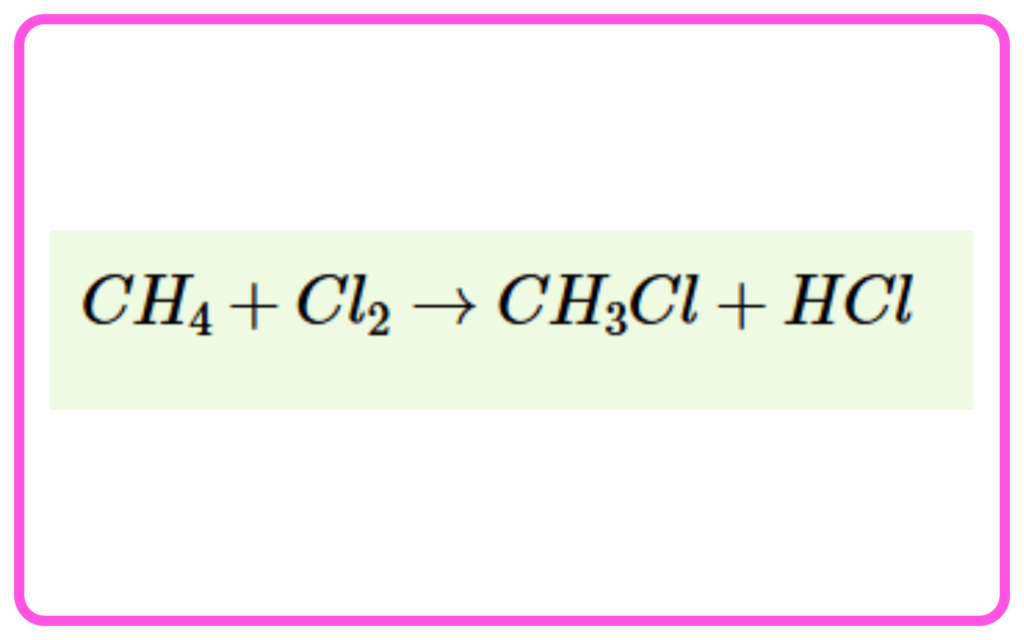
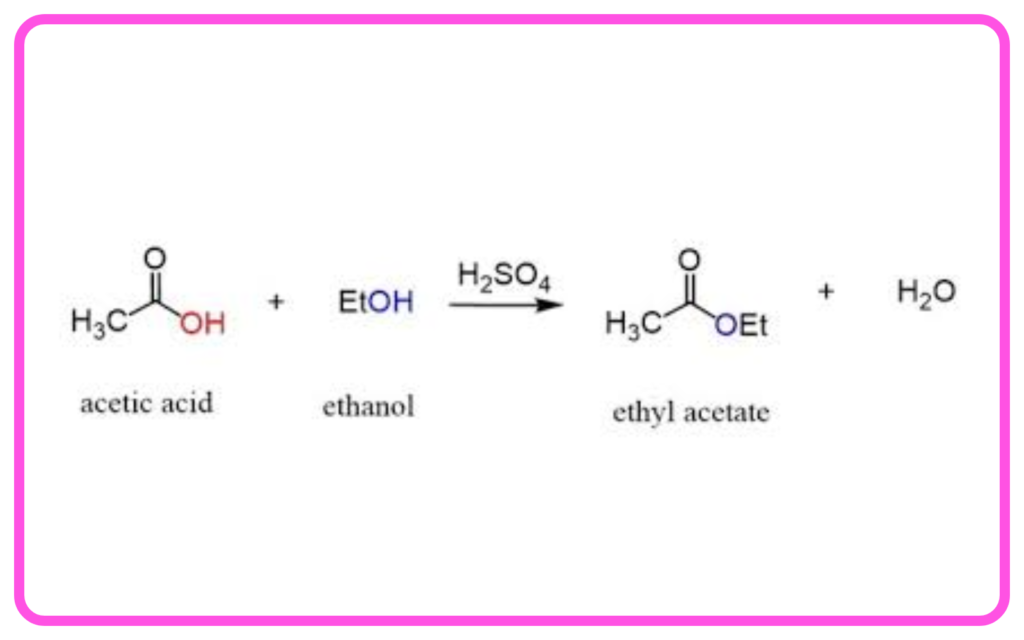
Esterification:
- Reaction between an alcohol and a carboxylic acid to form an ester and water.
- Esters have a fruity smell and are used in perfumes and flavorings.
- Example: Ethanol + Ethanoic Acid → Ethyl Acetate + Water.
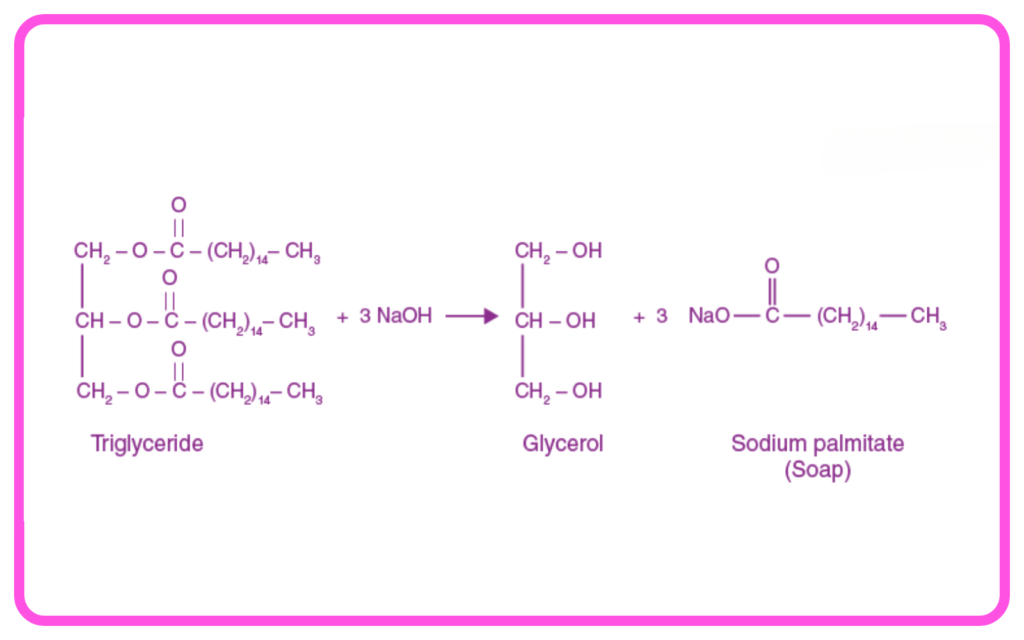
Saponification:
- Hydrolysis of esters (fats or oils) in the presence of a base (like NaOH) to produce soap and glycerol.
- Example: Triglyceride + Sodium Hydroxide → Soap + Glycerol.
Polymerization:
- Small carbon compounds (monomers) join together to form long chains (polymers).
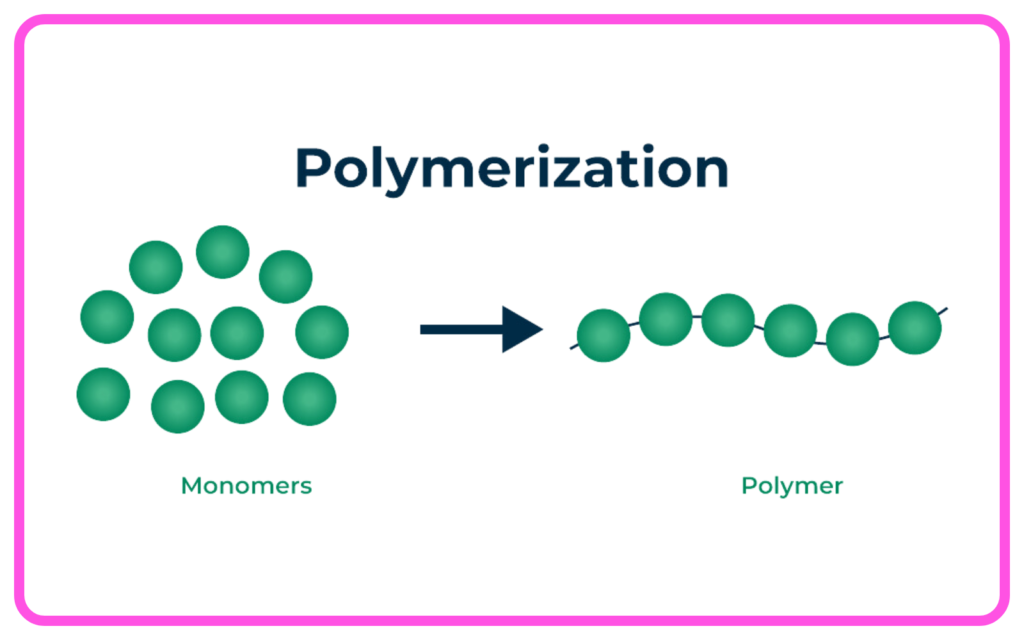
- Can be Addition Polymerization (like polyethylene) or Condensation Polymerization (like nylon).
Dehydration:
- Removal of water molecules from a compound, typically using a dehydrating agent like concentrated sulfuric acid.
- Example: Ethanol can be dehydrated to form ethene.
Let’s practice!

