Versatile Nature Of Carbon
Key Notes:
Tetravalency:
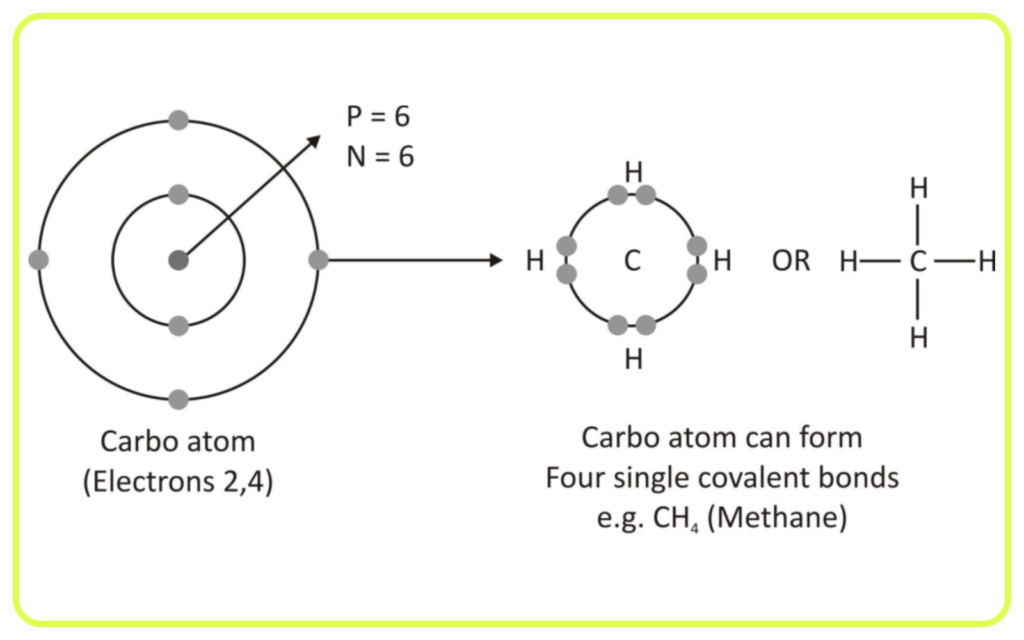
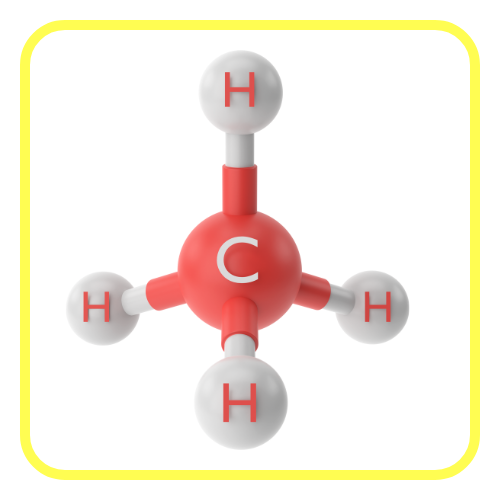
- Carbon has four valence electrons, which allows it to form four covalent bonds with other atoms (like hydrogen, oxygen, nitrogen, or even other carbon atoms).
- This ability to bond with multiple elements and itself is the foundation of its versatility.
Catenation:
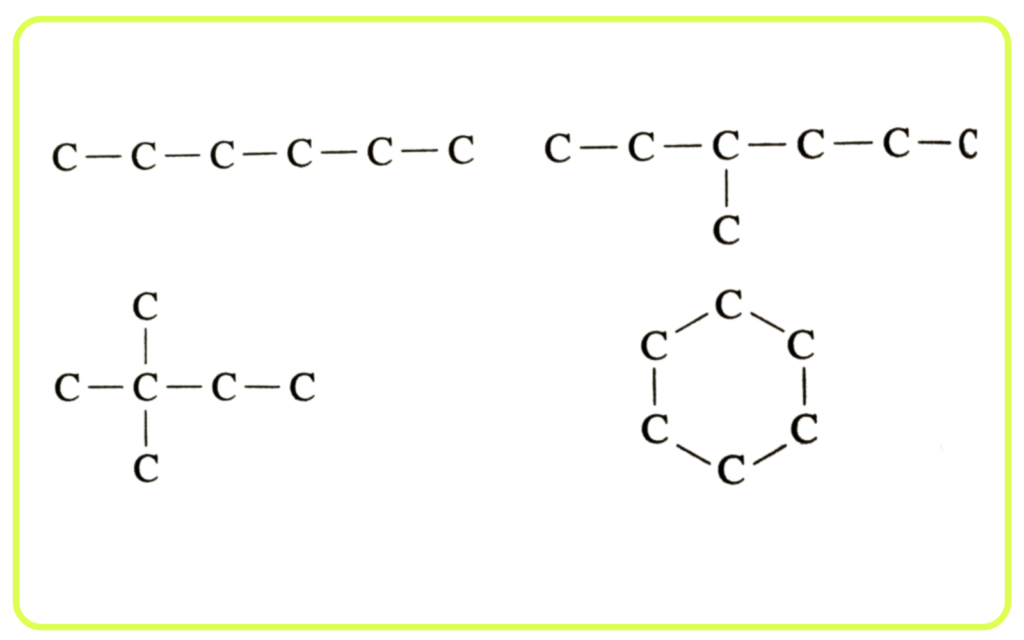
- Carbon can form long chains, branched structures, or rings by bonding with other carbon atoms.
- This unique property is called catenation and enables the formation of a vast number of organic compounds, including simple hydrocarbons and complex biomolecules.
Formation of Multiple Bonds:
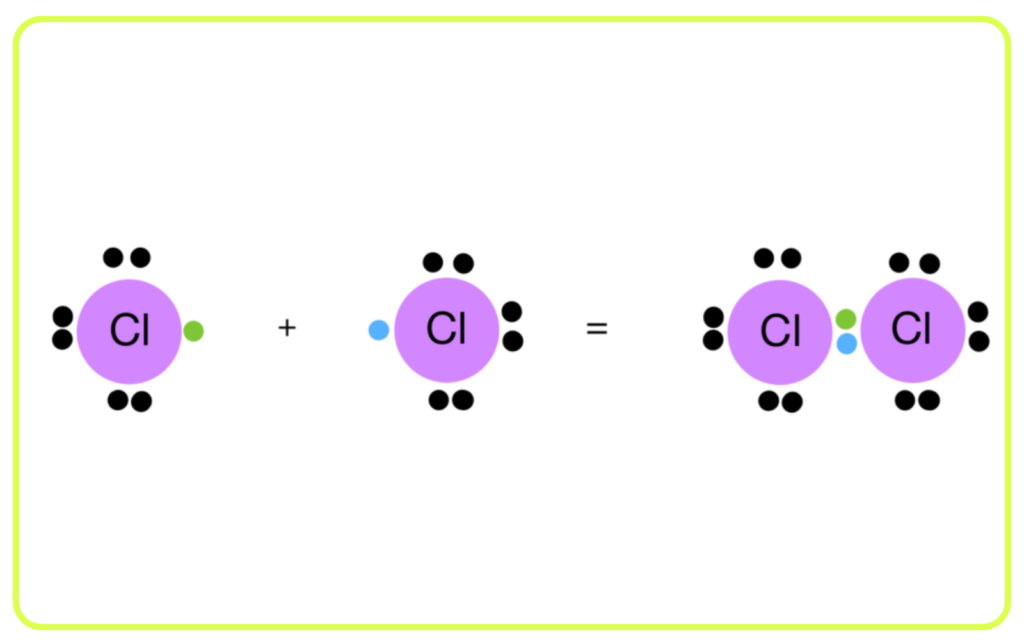
- Carbon can form single (C-C), double (C=C), and triple (C≡C) bonds with other carbon atoms.
- This results in a wide variety of compounds with different chemical properties, such as alkanes (single bonds), alkenes (double bonds), and alkynes (triple bonds).
Allotropes of Carbon:
- Carbon exists in different physical forms (allotropes) such as:
- Diamond: A hard, transparent structure where each carbon atom is bonded to four others, forming a 3D network.

- Graphite: A soft, opaque material where carbon atoms are arranged in layers with weak bonds between them, making it slippery and a good conductor of electricity.

- Fullerenes and Graphene: Newer forms with unique properties used in nanotechnology, electronics, and materials science.
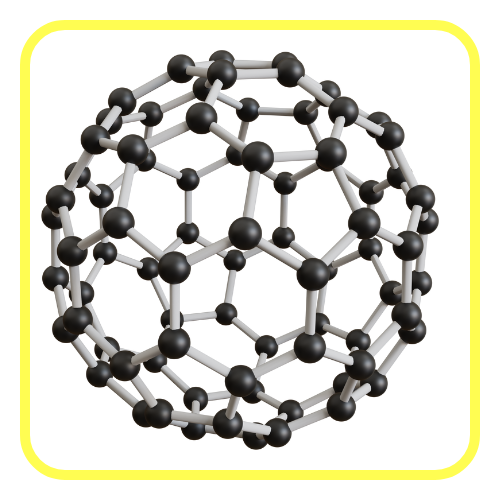
Diverse Compound Formation:
- Carbon forms a variety of compounds like hydrocarbons (e.g., methane, ethene, ethyne) and functional group-containing compounds (like alcohols, acids, and ketones).
- Organic chemistry, which is based on carbon compounds, plays a significant role in everyday life, including pharmaceuticals, fuels, plastics, and more.
Isomerism:
- Carbon compounds exhibit isomerism, meaning they can have the same molecular formula but different structural arrangements.

- This leads to diverse chemical and physical properties among compounds with the same elements.
Stable Bonds:
- The carbon-carbon bonds are strong and stable, contributing to the durability of organic molecules.
- This stability is essential for the structure of complex molecules like DNA and proteins, which are vital to life.

Importance in Life:
- Carbon is a fundamental building block of life. All living organisms are composed of carbon-based molecules, including carbohydrates, proteins, lipids, and nucleic acids.
- The versatility of carbon is what allows for the complexity and diversity of life forms on Earth.
Let’s practice!

