Occurrence Of Metals
Key Notes:
Natural Occurrence of Metals:
- Metals are found in the Earth’s crust in various forms, primarily as ores.

- Some metals are found in their native state (uncombined form) due to their low reactivity, like gold, silver, and platinum.

- Most metals occur as compounds in minerals and ores, usually combined with non-metals like oxygen, sulfur, or carbonates.

Classification of Metals Based on Reactivity:
- Highly reactive metals (e.g., sodium, potassium, calcium) are found in the form of salts such as chlorides, carbonates, or sulfates.

- Moderately reactive metals (e.g., zinc, iron, lead) are typically found in oxide or sulfide ores.

- Low reactive metals (e.g., gold, silver, platinum) are often found in their free or elemental state.

Minerals and Ores:
- A mineral is a naturally occurring substance that contains a metal or a metal compound.

- An ore is a type of mineral that contains enough of a metal to make extraction economically viable.
- Example: Bauxite (Al₂O₃·nH₂O) is the ore of aluminum, and hematite (Fe₂O₃) is the ore of iron.

Occurrence of Common Metals:
- Iron: Found as hematite (Fe₂O₃), magnetite (Fe₃O₄), and limonite (Fe₂O₃·nH₂O).


- Aluminum: Found as bauxite ore.

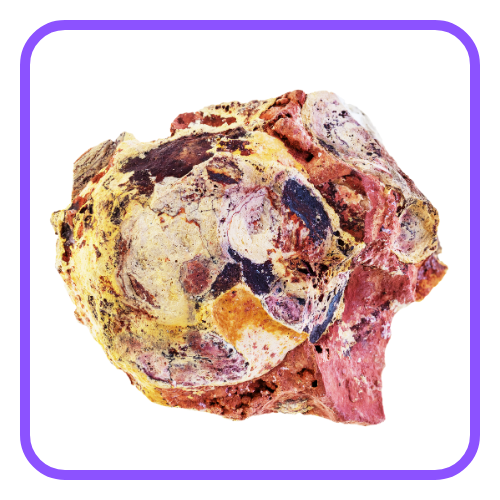
- Copper: Occurs as sulfides (e.g., chalcopyrite – CuFeS₂) and carbonates (e.g., malachite – Cu₂(CO₃)(OH)₂).


- Zinc: Found as zinc blende (ZnS) and calamine (ZnCO₃).


Extraction of Metals:
- Metals are extracted from their ores through chemical and physical processes like reduction, electrolysis, or pyrometallurgical processes.
- For example, iron is extracted from its ore in a blast furnace, while aluminum is extracted from bauxite using electrolysis.
Important Terms:
- Gangue: The impurities (such as sand and rocks) that are found along with the ore.
- Metallurgy: The process of extracting metals from their ores and refining them for use.

Economic Importance:
- The abundance, location, and ease of extraction of metals affect their economic importance. Some countries have more reserves of certain metals, making mining and export of those metals crucial to their economies.
Let’s practice!

