Corrosion
Key Notes
Corrosion:
- Corrosion is the gradual deterioration of metals due to their reaction with environmental factors such as oxygen, moisture, and pollutants.
- Example: Rusting of iron.

Causes of Corrosion:
- Presence of Moisture: Water in the environment facilitates the oxidation of metals.

- Oxygen: Combines with metals to form oxides.

- Pollutants: Gases like sulfur dioxide and carbon dioxide accelerate corrosion.

Examples of Corrosion:
- Iron: Rusts to form hydrated ferric oxide (Fe₂O₃·xH₂O).

- Copper: Forms a green layer of basic copper carbonate (patina).

- Silver: Tarnishes to form silver sulfide (Ag₂S).

- Aluminium: Forms a protective layer of aluminium oxide (Al₂O₃).

Rusting of Iron:
- Reaction: 4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃, which dehydrates to Fe₂O₃·xH₂O (rust).
- Rusting is a specific type of corrosion that weakens iron structures.

Factors Affecting Corrosion:
- Humidity: High moisture levels increase corrosion.

- Temperature: Higher temperatures accelerate chemical reactions.

- Impurities: Saltwater and acidic pollutants enhance corrosion rates.
Prevention of Corrosion:
- Coating and Painting: Creates a protective barrier against moisture and air.

- Galvanization: Coating iron with a layer of zinc.

- Alloying: Mixing metals to improve corrosion resistance (e.g., stainless steel).
- Electroplating: Depositing a layer of a less reactive metal on the surface.
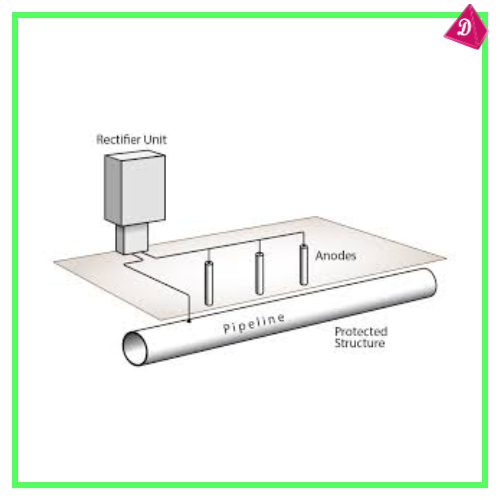
- Cathodic Protection: Using a more reactive metal as a sacrificial anode to protect the main metal.
- Greasing or Oiling: Prevents moisture contact with the metal surface.

Impact of Corrosion:
- Weakens metal structures, reducing their strength and durability.
- Causes economic loss due to the replacement and maintenance of corroded materials.
- Leads to safety hazards, especially in bridges, buildings, and pipelines.
Let’s practice!

