Extraction Of Metals From The Ores
Key Notes:
Definition of Ore:
- Ores are naturally occurring rocks or minerals containing sufficient quantities of metal for economic extraction.

Stages in Metal Extraction:
- Concentration of Ore: Removal of impurities (gangue) to increase the metal concentration.
- Methods include:
- Froth Flotation: For sulfide ores like copper.
- Magnetic Separation: For ores like hematite (iron).
- Gravity Separation: For denser ore particles.
- Methods include:
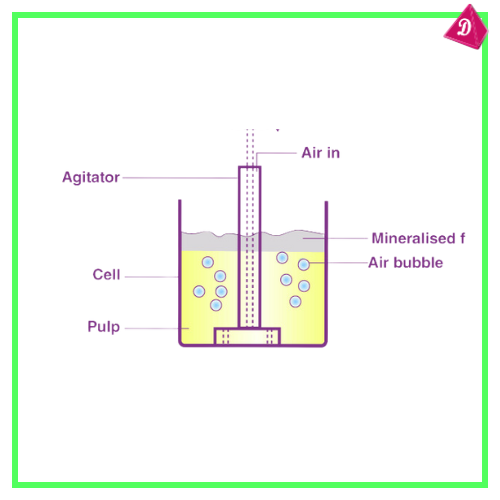
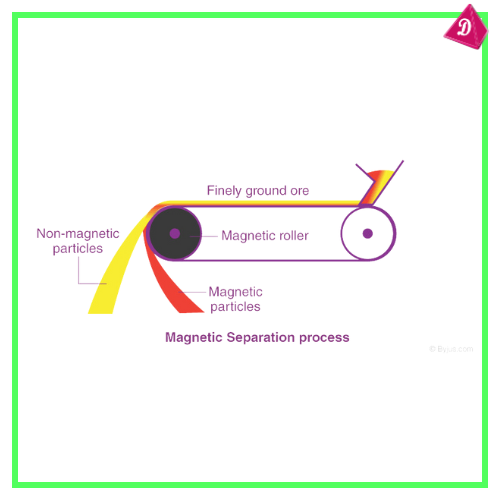
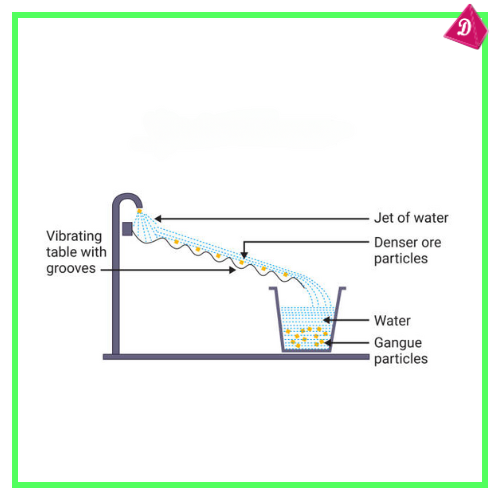
- Extraction of Metal:
- Metals are extracted depending on their position in the reactivity series.
- Purification of Metal: Removing impurities to obtain pure metal.
Classification Based on Reactivity:
- Highly Reactive Metals:
- Extracted via Electrolysis.
- Example: Aluminium from bauxite (Al₂O₃·2H₂O).
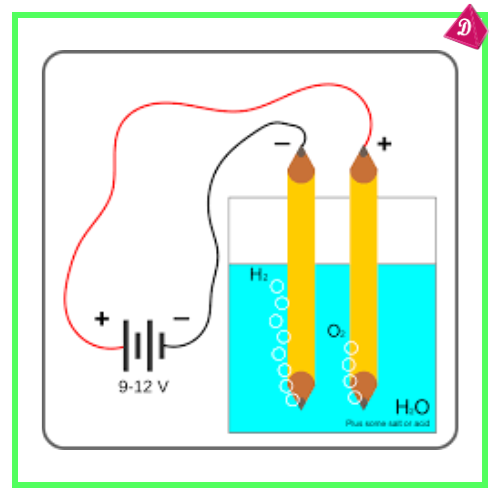

- Moderately Reactive Metals:
- Reduced using carbon or carbon monoxide.
- Example: Iron from hematite (Fe₂O₃) using a blast furnace.

- Less Reactive Metals:
- Found in the free state or reduced by simple methods.
- Example: Gold, silver.

Common Methods of Extraction:
- Reduction by Carbon:
- Used for metals like iron.
- Reaction: Fe₂O₃ + 3CO → 2Fe + 3CO₂.
- Electrolytic Reduction:
- Used for metals like sodium, potassium, and aluminium.
- Reaction: 2Al₂O₃ → 4Al + 3O₂ (in a molten state).
- Thermal Decomposition:
- For less stable ores.
- Example: 2HgO → 2Hg + O₂.
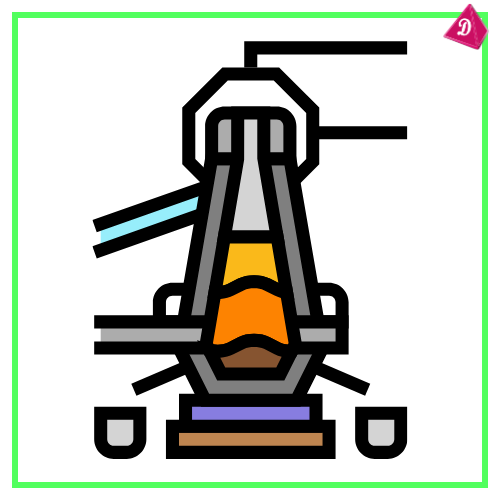
Blast Furnace Process for Iron:
- Inputs: Iron ore, coke, and limestone.
- Outputs: Molten iron and slag (waste material).
- Reaction:
- Formation of CO: C + O₂ → CO₂; CO₂ + C → 2CO.
- Reduction of iron ore: Fe₂O₃ + 3CO → 2Fe + 3CO₂.

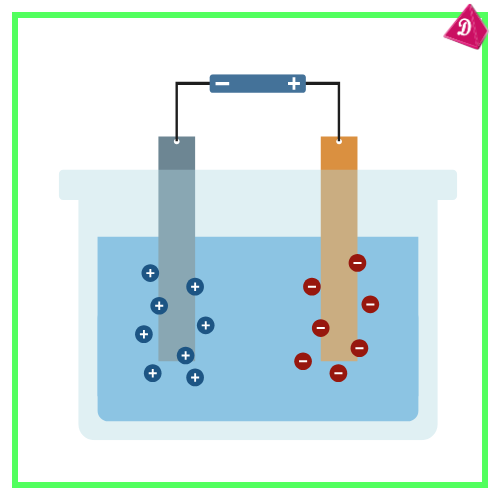
Purification of Metals:
- Impure metals are purified using Electrolytic Refining.
- Example: Copper refining.
- Impure copper is the anode; pure copper collects at the cathode.
Environmental Considerations:
- Mining and extraction lead to habitat destruction, pollution, and energy use.
- Recycling and sustainable methods can minimize environmental impact.

Let’s practice!

