More About Salts
Key Notes:
Definition of Salts:
- Salts are ionic compounds formed by the neutralization reaction between an acid and a base.

- They consist of positive ions (cations) and negative ions (anions).
Formation of Salts:
- Neutralization Reaction: When an acid reacts with a base, it forms salt and water.
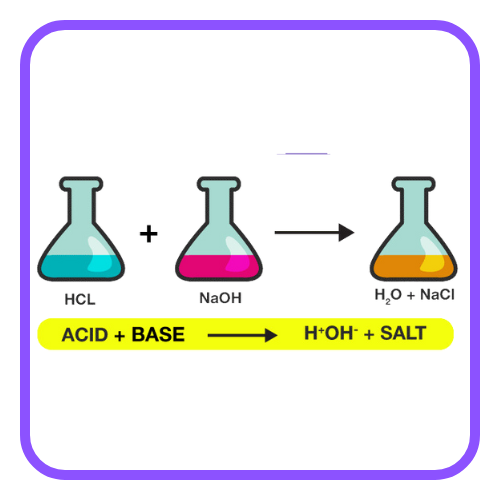
- Reaction with Metals: Metals react with acids to form salts and hydrogen gas.
- Example: Zn+H2SO4→ZnSO4+H2
Types of Salts:
- Normal Salts: Formed when all the hydrogen ions in an acid are replaced by metal ions.
- Example: Sodium chloride (NaCl)

- Acid Salts: Formed when only part of the hydrogen ions in an acid are replaced by metal ions.
- Example: Sodium bisulfate (NaHSO₄)

- Basic Salts: Formed by the partial replacement of hydroxide ions in a base with an acid radical.
- Example: Basic lead carbonate (Pb(OH)₂CO₃)

Properties of Salts:
- Solubility: Most salts are soluble in water, but some, like barium sulfate (BaSO₄), are insoluble.
- Electrical Conductivity: When dissolved in water, salts dissociate into ions, making the solution conductive.
- Melting and Boiling Points: Salts generally have high melting and boiling points.
pH of Salt Solutions:
- Neutral Salts: Formed from strong acids and strong bases; their solutions are neutral (pH = 7).
- Acidic Salts: Formed from a strong acid and a weak base; their solutions are acidic (pH < 7).
- Basic Salts: Formed from a weak acid and a strong base; their solutions are basic (pH > 7).

Common Uses of Salts:
- Table Salt (NaCl): Used in cooking and food preservation.

- Baking Soda (NaHCO₃): Used in baking as a leavening agent.

- Epsom Salt (MgSO₄): Used in bath salts and as a laxative.

- Bleaching Powder (Ca(OCl)₂): Used for disinfection and water purification.

Crystallization of Salts:
- Salts can be crystallized from their aqueous solutions by evaporating the water, leading to the formation of solid crystals.
Environmental Impact:
- Some salts can cause environmental issues, such as soil salinization, which affects agriculture and plant growth.

Let’s practice!

