How strong are acid or base solutions
Key Notes:
Strength of Acids and Bases:
- The strength of acids or bases refers to their ability to dissociate into ions in water.
- Strong acids (e.g., HCl, H₂SO₄) and strong bases (e.g., NaOH, KOH) dissociate completely in water.


- Weak acids (e.g., CH₃COOH) and weak bases (e.g., NH₃) dissociate partially in water.


pH Scale:
The pH scale measures the acidity or basicity of a solution.
It ranges from 0 to 14, where:
- pH < 7: Acidic solutions.
- pH = 7: Neutral solutions.
- pH > 7: Basic solutions.
Lower pH values indicate stronger acids, while higher values indicate stronger bases.

Indicators of Strength:
- Indicators, such as litmus paper, phenolphthalein, and methyl orange, change color depending on the pH of the solution.
- Universal indicators and pH meters provide more precise measurements of pH.

Relation to Hydrogen Ion Concentration:
- pH = -log[H⁺], where [H⁺] is the concentration of hydrogen ions in moles per liter.
- A small change in pH corresponds to a large change in hydrogen ion concentration because the pH scale is logarithmic.
pOH and Relationship to pH:
- pOH measures hydroxide ion concentration: pOH = -log[OH⁻].
- Relationship: pH + pOH = 14 (at 25°C).
Neutralization and Titration:
- Strong acids neutralize strong bases to form salt and water.
- Titration is a method used to determine the concentration of an acid or base by adding a solution of known concentration until neutralization.

Examples of Applications:
- pH in everyday life: Maintaining body pH, testing soil pH for agriculture, and determining the acidity of food and beverages.
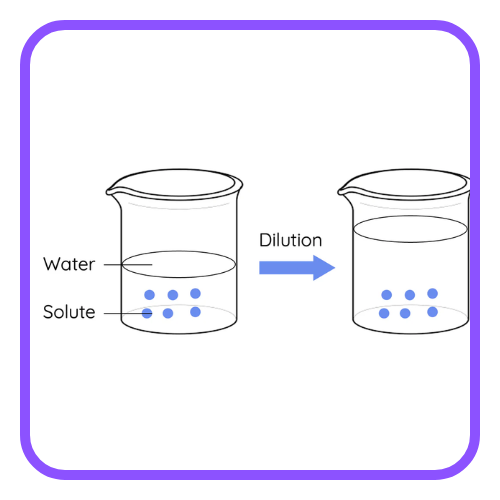
Effect of Dilution:
- Diluting an acidic or basic solution decreases the concentration of H⁺ or OH⁻ ions, thus increasing or decreasing the pH closer to neutral (7).
Buffer Solutions:
- Buffers resist changes in pH upon the addition of small amounts of acid or base.
- They are crucial in biological systems to maintain stable pH.
Environmental Relevance:
- Acid rain has a low pH due to dissolved gases like SO₂ and NO₂

- Maintaining proper pH levels in water bodies is vital for aquatic life.

Let’s practice!

