What Do All Acids And All Bases Have In Common?
Key Notes:
Acids and Bases: Definitions
- Acids: Substances that release hydrogen ions (H⁺) when dissolved in water.
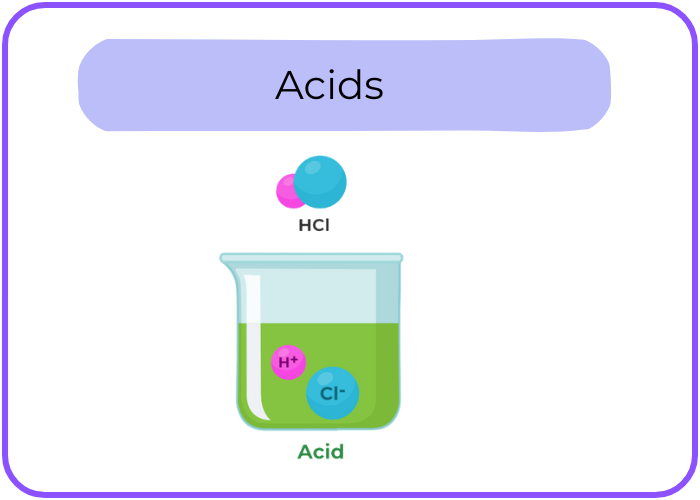
- Bases: Substances that release hydroxide ions (OH⁻) when dissolved in water.
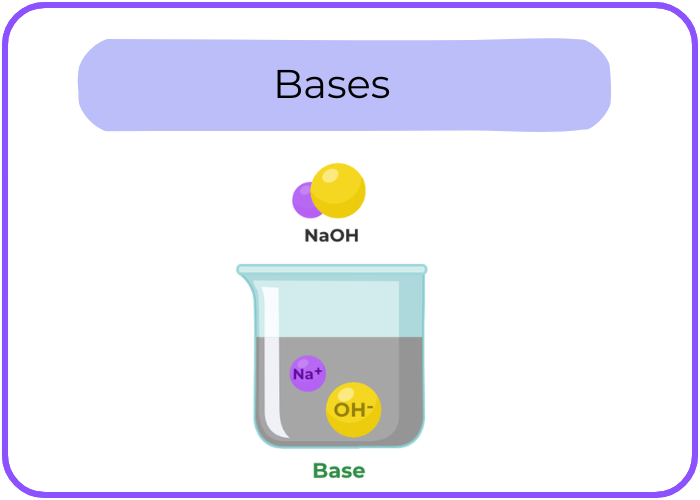
Common Properties
- Electrolytes: Both acids and bases can conduct electricity in aqueous solutions because they dissociate into ions.
- Taste:
- Acids have a sour taste (e.g., citric acid in lemon).

- Bases have a bitter taste and a slippery feel (e.g., soap).

- Indicator Reactions:
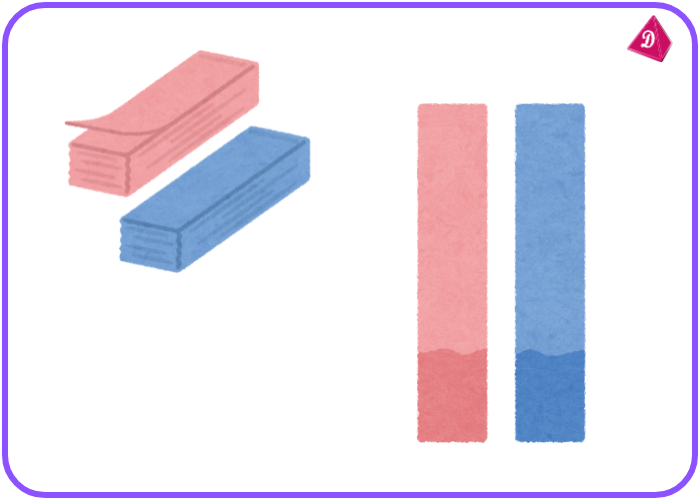
- Litmus Paper: Acids turn blue litmus paper red; bases turn red litmus paper blue.
- pH Scale: Both acids and bases can be measured on the pH scale, with acids below 7 and bases above 7.

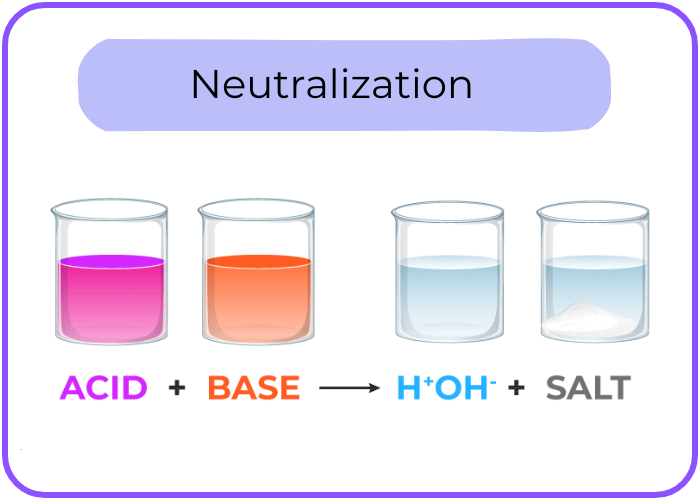
Neutralization Reaction
- When acids and bases react together, they undergo a neutralization reaction, forming water and a salt.
- General Equation: Acid + Base → Salt + Water
Ionization in Water
- Acids ionize in water to produce H⁺ ions.
- Bases ionize in water to produce OH⁻ ions.
- Both acids and bases affect the concentration of these ions in water, influencing the solution’s pH.
Corrosiveness
- Both acids and bases can be corrosive, meaning they can cause damage to materials and tissues.

Role in Chemical Reactions
- Acids and bases are commonly involved in chemical reactions, including acid-base reactions, which are essential in various biological and industrial processes.
Presence in Everyday Life
- Acids are found in foods (e.g., vinegar, citrus fruits), while bases are found in cleaning products (e.g., baking soda, soap).

Brønsted-Lowry Theory
- According to this theory:
- Acid: A substance that can donate a proton (H⁺).
- Base: A substance that can accept a proton (H⁺).
Let’s practice!

