Understanding The Chemical Properties Of Acids, Bases, And Salts
key notes :-
Acids
- Definition: Acids are substances that release hydrogen ions (H⁺) when dissolved in water. They have a pH less than 7.
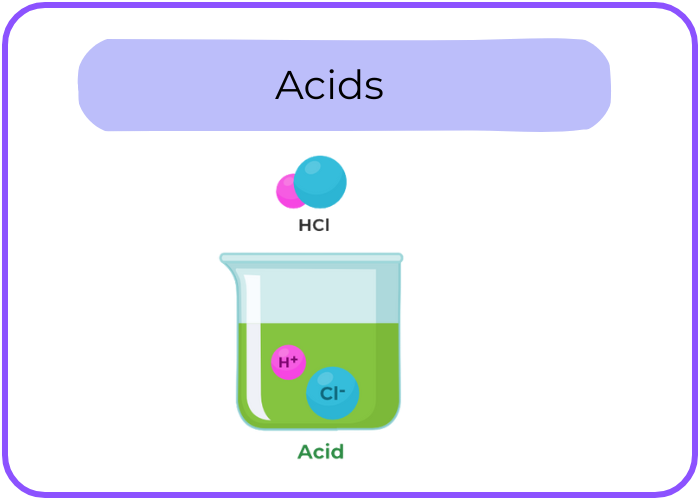
- Taste: Acids generally have a sour taste (e.g., lemon juice, vinegar).

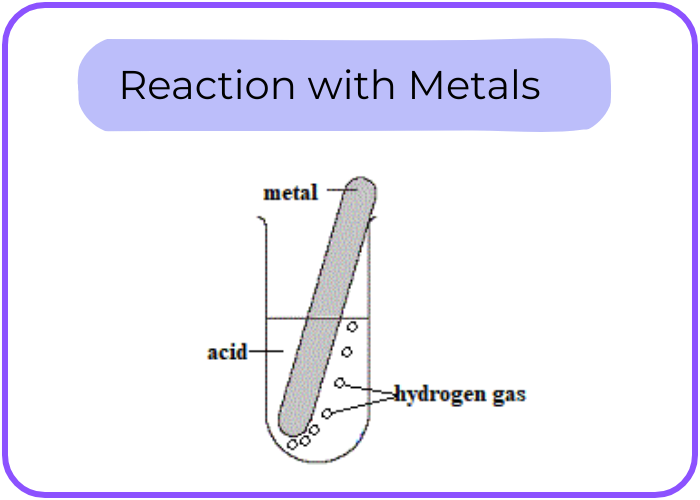
- Reaction with Metals: Acids react with certain metals (e.g., Zn, Mg) to produce hydrogen gas and a salt.
- Example: Zn +2HCl → ZnCl2 +H2
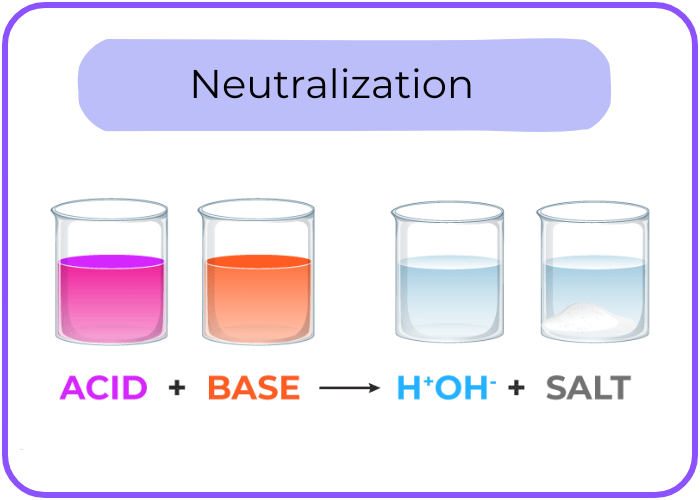
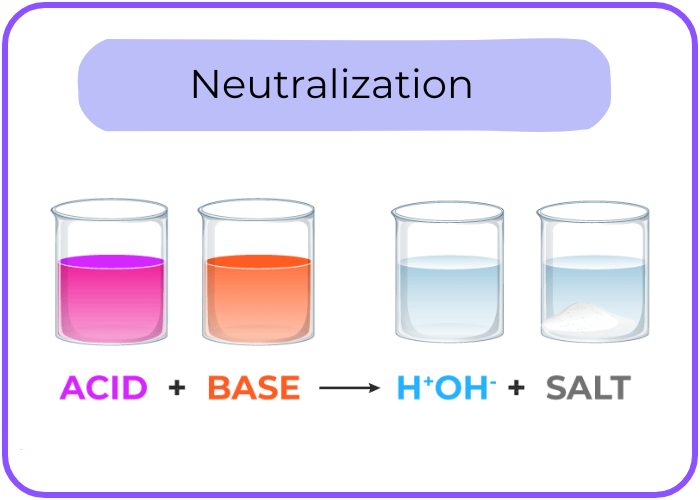
- Reaction with Bases (Neutralization): Acids react with bases to form water and a salt.
- Example: HCl + NaOH → NaCl + H2O
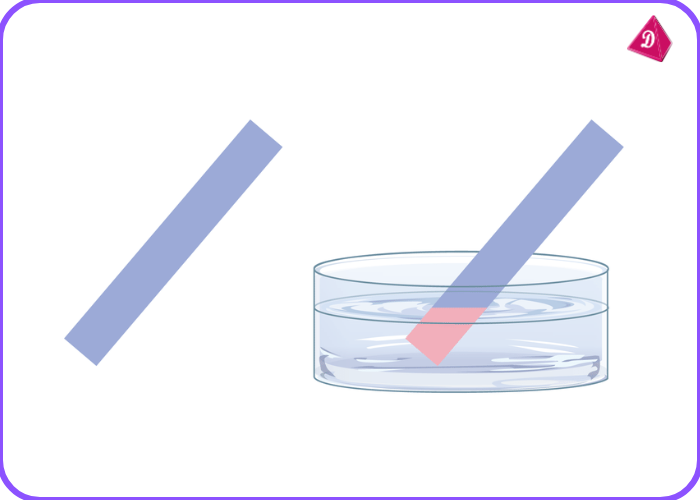
- Indicators: Acids turn blue litmus paper red.
Bases
- Definition: Bases are substances that release hydroxide ions (OH⁻) when dissolved in water. They have a pH greater than 7.
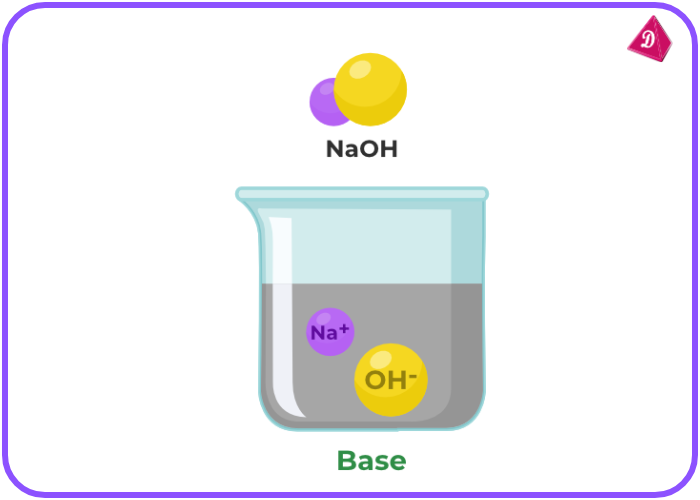
- Taste and Feel: Bases typically have a bitter taste and feel slippery (e.g., soap, baking soda).

- Reaction with Acids (Neutralization): Bases neutralize acids to produce water and a salt.
- Example: NaOH + HCl → NaCl + H2O
- Reaction with Ammonium Salts: Bases react with ammonium salts to release ammonia gas.
- Example: NH4Cl + NaOH → NH3 +H2O + NaCl
- Indicators: Bases turn red litmus paper blue.
Salts
- Definition: Salts are ionic compounds formed from the neutralization reaction between an acid and a base.

- Formation: Salt formation can occur through various reactions:
- Acid + Base: Produces salt and water (neutralization).
- Acid + Metal: Produces salt and hydrogen gas.
- Acid + Metal Carbonate: Produces salt, water, and carbon dioxide.
- Examples of Salts: Sodium chloride (NaCl), calcium carbonate (CaCO₃), potassium nitrate (KNO₃).

- pH of Salts: The pH of a salt solution can be acidic, basic, or neutral, depending on the strengths of the parent acid and base.
pH Scale
- Definition: The pH scale measures the acidity or basicity of a solution. It ranges from 0 to 14.

- pH < 7: Acidic
- pH = 7: Neutral
- pH > 7: Basic
- Importance: pH is crucial in various chemical processes, biological systems, and industrial applications.
Indicators
- Definition: Indicators are substances that change color in the presence of an acid or base.
- Common Indicators:
- Litmus: Red in acid, blue in base.
- Phenolphthalein: Colorless in acid, pink in base.

- Methyl Orange: Red in acid, yellow in base.

Applications of Acids, Bases, and Salts
- Acids: Used in food preservation, cleaning agents, and manufacturing (e.g., sulfuric acid in batteries).
- Bases: Used in soap production, baking, and antacid tablets.

- Salts: Used in cooking, fertilizers, and as preservatives.

Safety Precautions
- Handling Acids and Bases: Always wear protective gear (gloves, goggles) when handling strong acids or bases. Avoid direct contact with skin and eyes.

- Storage: Acids and bases should be stored in properly labeled containers and kept away from each other to prevent accidental reactions.
Let’s practice!

