Valence Bond Theory-Hybridisation
Key Notes:
Introduction to Valence Bond Theory (VBT)
- Valence Bond Theory explains how atoms combine to form chemical bonds by overlapping atomic orbitals.
- Developed by Linus Pauling and John C. Slater.
Key Concepts of VBT
- Atomic Orbital Overlap:
- Bonds are formed when atomic orbitals of two atoms overlap.
- The greater the overlap, the stronger the bond.
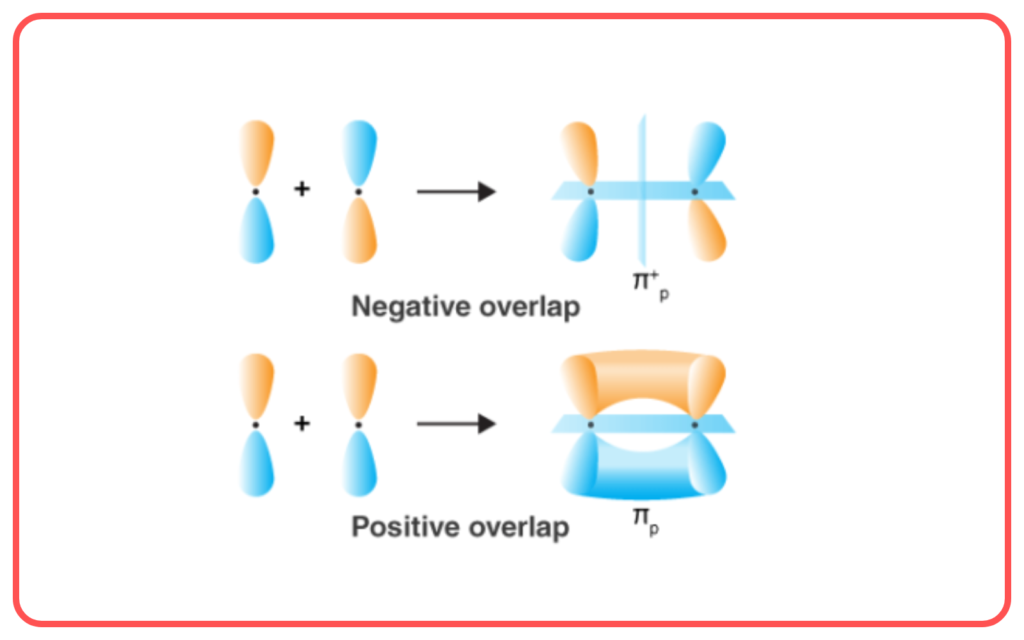
- Types of Overlap:
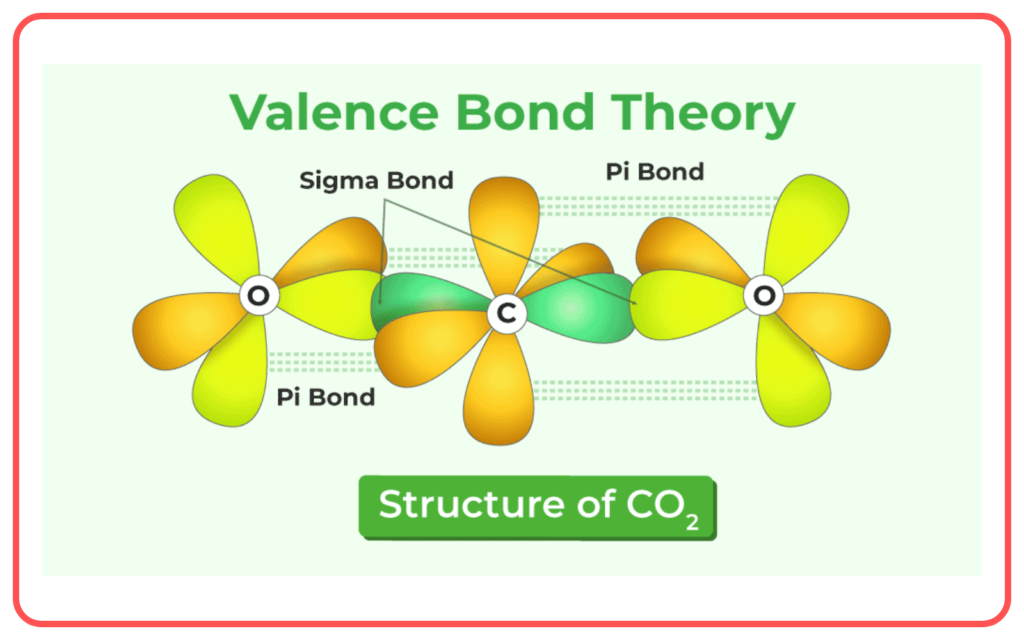
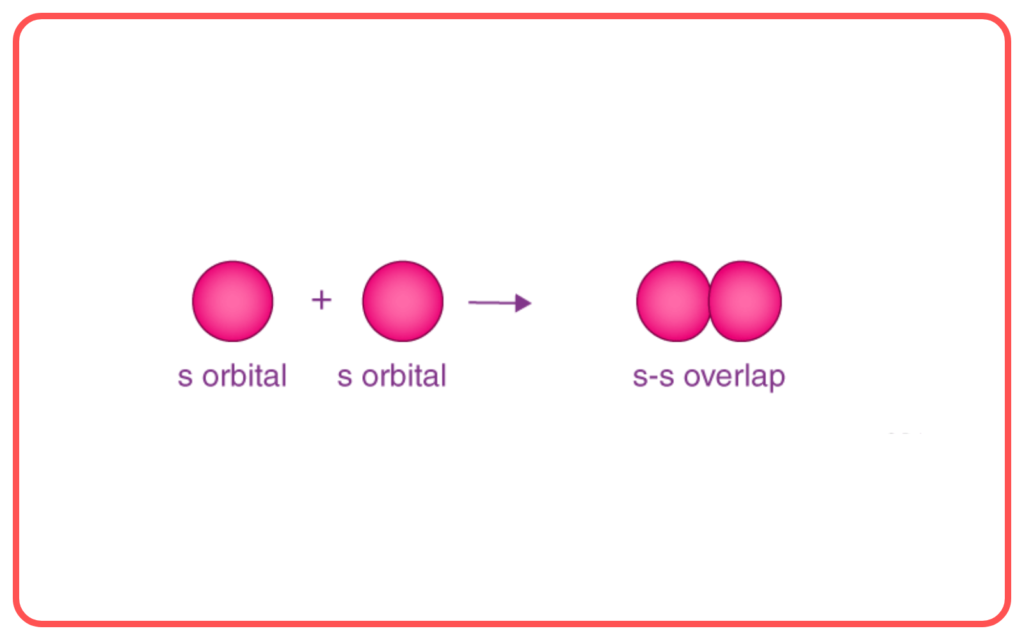
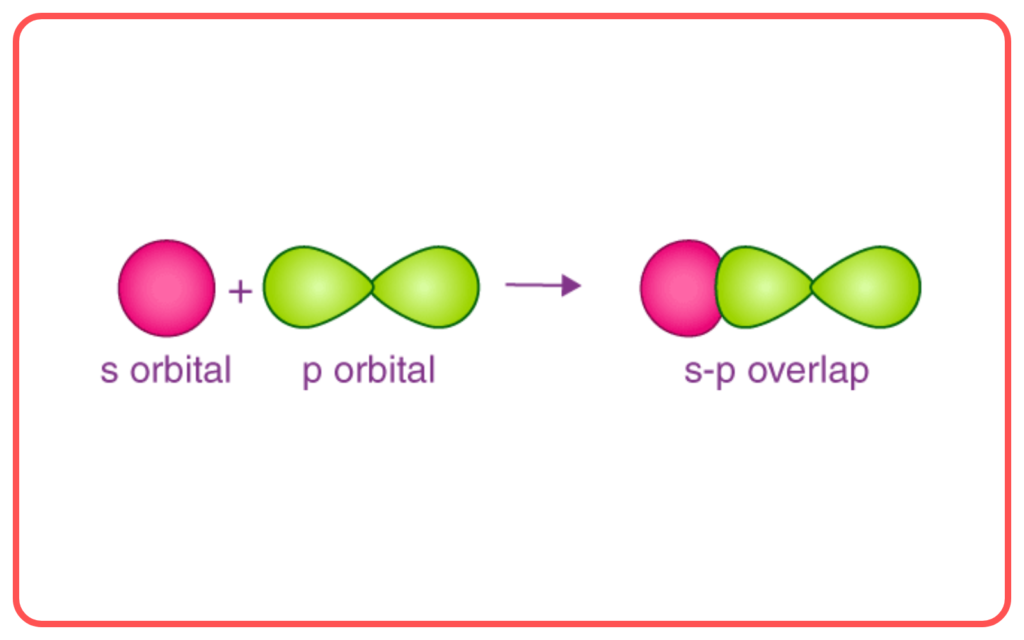
- Sigma (σ\sigma) Bond: Formed by the end-to-end overlap of orbitals (e.g., s−s, s−p, p−p).
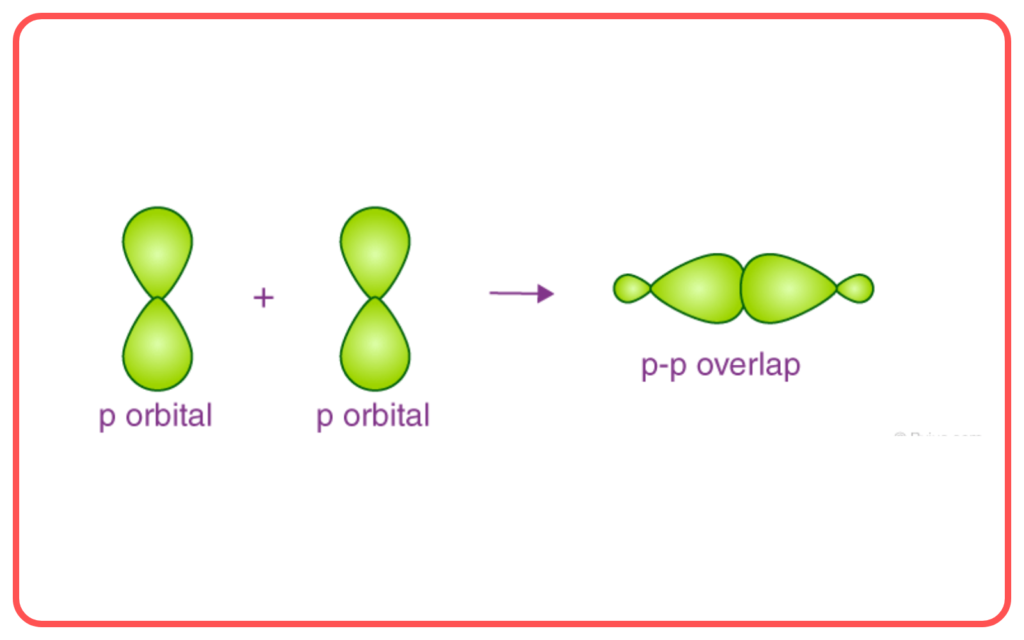
- Pi (π) Bond: Formed by the side-to-side overlap of p-orbitals.
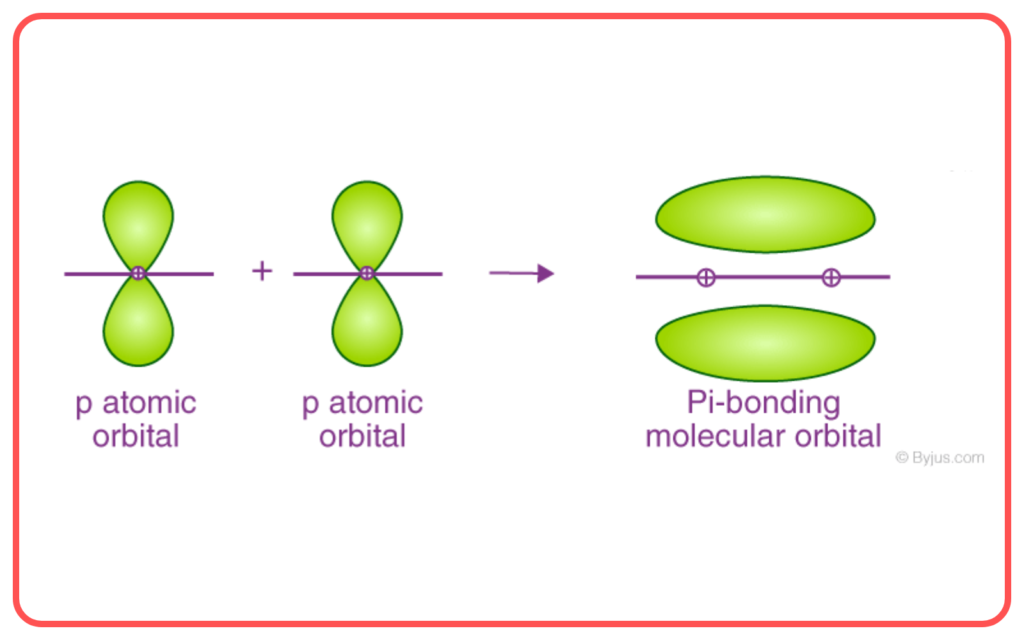
- Bond Strength:
- σ\sigma-bonds are stronger than π\pi-bonds due to greater overlap.
- Directionality:
- Covalent bonds are directional and depend on the orientation of the orbitals.
Introduction to Hybridization
- Hybridization is the mixing of atomic orbitals in an atom to form new hybrid orbitals of equal energy.
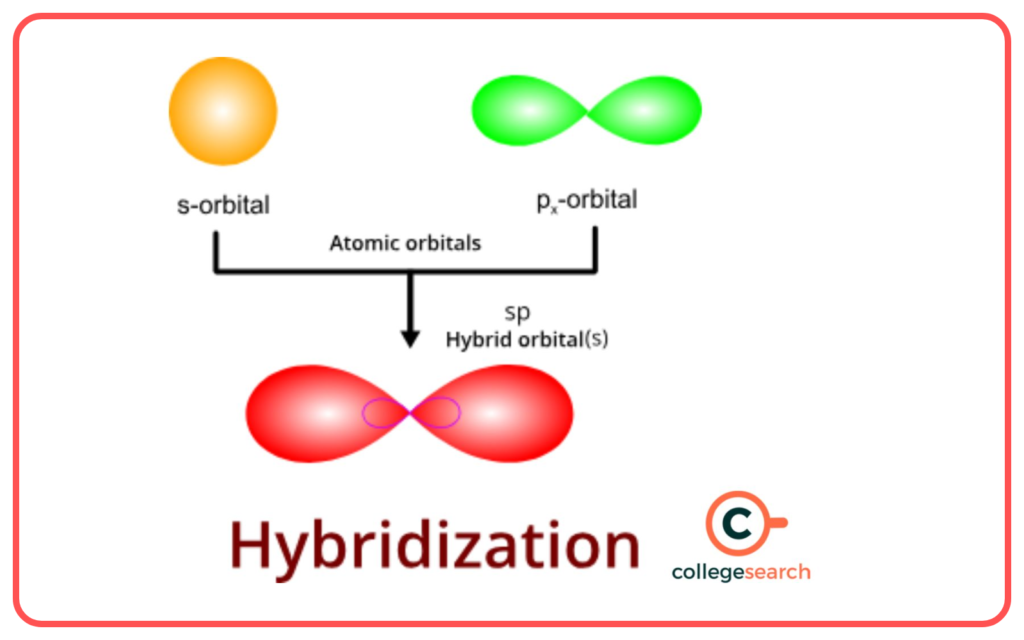
- Proposed to explain molecular shapes and bond angles.
Key Features of Hybridization
- Mixing of Orbitals:
- Involves combining ss, pp, and sometimes dd-orbitals.
- Hybrid Orbitals:
- Formed orbitals have the same energy and are used for bonding.
- Types of Hybridization:
- Depends on the number and type of orbitals involved.
Types of Hybridization and Examples
| Type | Orbitals Involved | Geometry | Bond Angle | Examples |
|---|---|---|---|---|
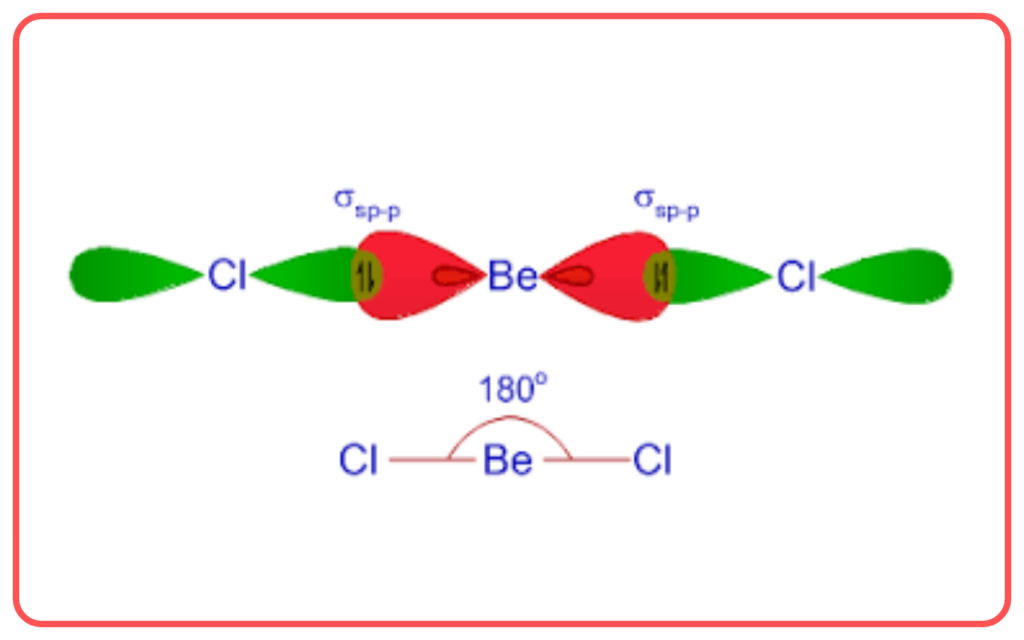
| sp | 1 s+ 1 p | Linear | 180° | BeCl₂ |
| sp² | 1 s + 2 p | Trigonal planar | 120° | BF₃, C₂H₄ |
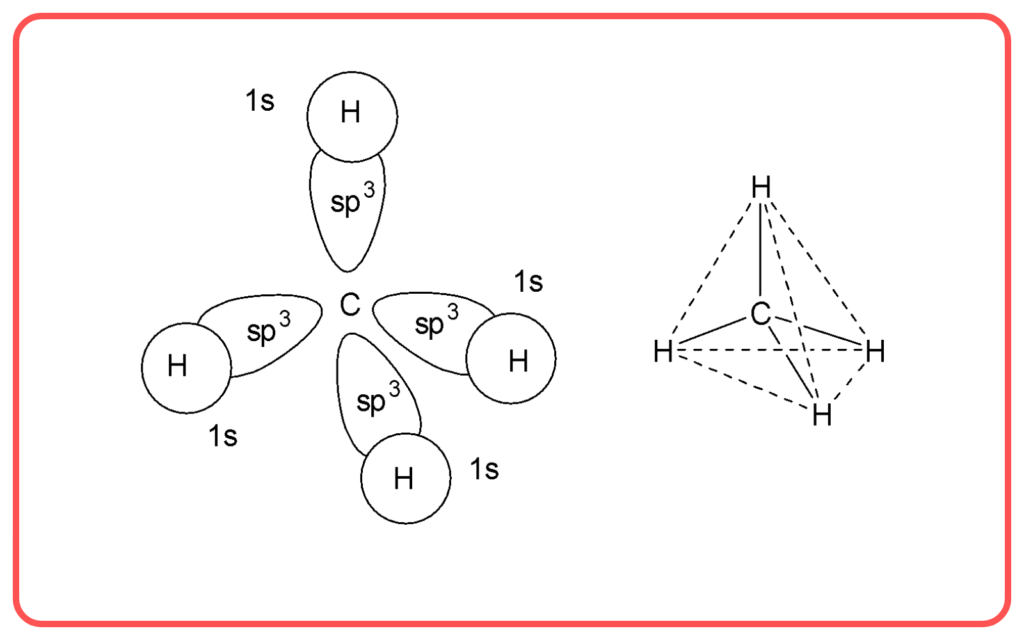
| sp³ | 1 s + 3 p | Tetrahedral | 109.5° | CH₄, NH,₃ H₂O |
| sp³d | 1 s + 3 p + 1 d | Trigonal bipyramidal | 90°,120° | PCl₅ |
| sp³d² | 1 s + 3 p + 2 d | Octahedral | 90° | SF₆ |
Steps to Determine Hybridization
- Count the Regions of Electron Density:
- Bonds (single, double, or triple) + lone pairs around the central atom.
- Match to Hybridization:
- 2 regions → sp.
- 3 regions → sp²
- 4 regions → sp³
Examples of Hybridization
- Methane (CH₄):
- Central atom: Carbon.
- Regions of electron density: 4.
- Hybridization: sp³
- Shape: Tetrahedral.
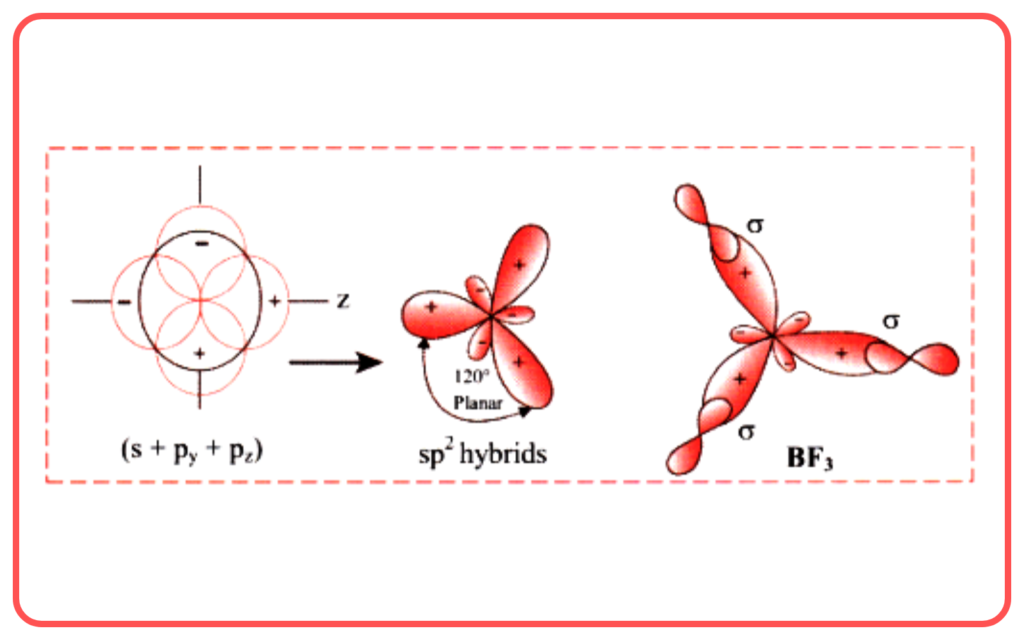
- Boron Trifluoride (BF₃):
- Central atom: Boron.
- Regions of electron density: 3.
- Hybridization: sp²
- Shape: Trigonal planar.
- BeCl₂:
- Central atom: Beryllium.
- Regions of electron density: 2.
- Hybridization: sp.
- Shape: Linear.
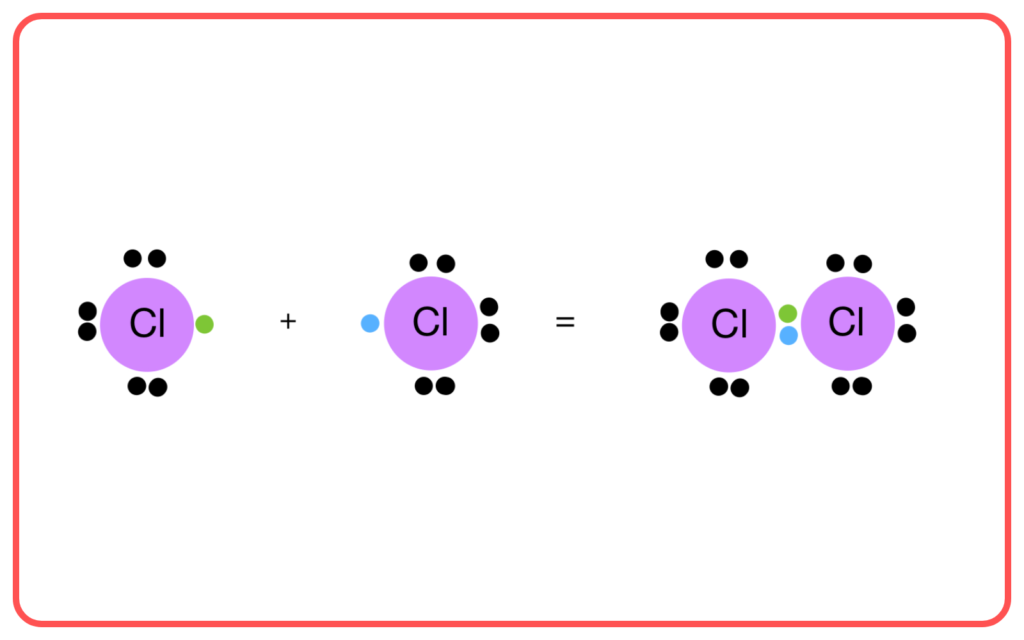
Importance of VBT and Hybridization
- Explains Bond Formation:
- Describes how atoms share electrons to form stable molecules.
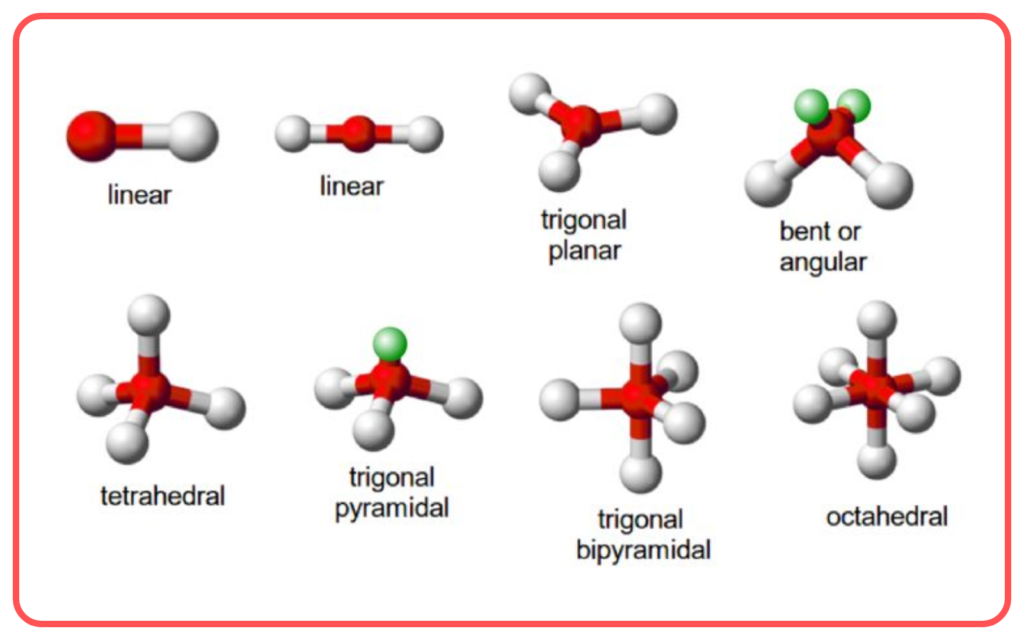
- Predicts Molecular Shapes:
- Hybridization provides insights into geometry and bond angles.
- Supports Chemical Reactivity:
- Helps in understanding the stability and reactivity of molecules.
Limitations of Valence Bond Theory
- Doesn’t Explain All Properties:
- Fails to explain magnetic properties of some molecules (e.g., O₂).
- No Energy Insights:
- Provides no quantitative details about bond energies.
- Lacks Molecular Orbital Theory Concepts:
- Overlooks delocalization of electrons (e.g., in benzene).
Conclusion
Valence Bond Theory and Hybridization are crucial for understanding how atoms form bonds and determine molecular shapes. While VBT explains bonding through orbital overlap, hybridization provides a detailed view of molecular geometry and bond angles.
Let’s practice!

