Valence Bond Theory
Key Notes:
1.Definition of Covalent Bond
A covalent bond is formed when two atoms share electrons, and this bond is strongest when their atomic orbitals overlap. The electrons involved must have opposite spins to form a stable bond.
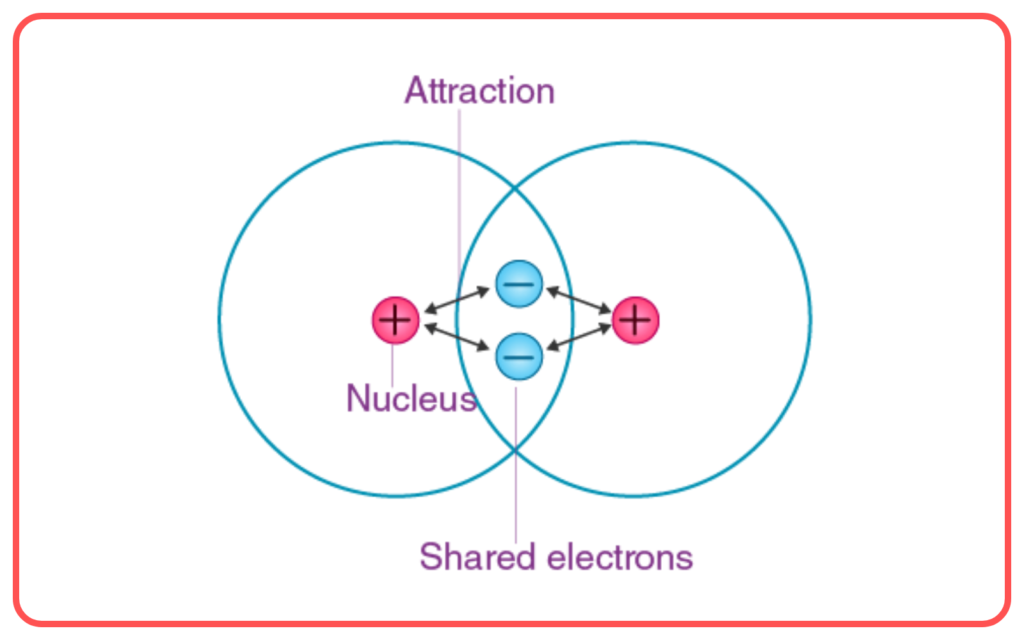
2. Orbital Overlap
The strength of a covalent bond depends on the extent of overlap between atomic orbitals. The greater the overlap, the stronger the bond. This overlap leads to the formation of molecular orbitals, where electrons are shared between atoms.

3. Types of Bonds in VBT
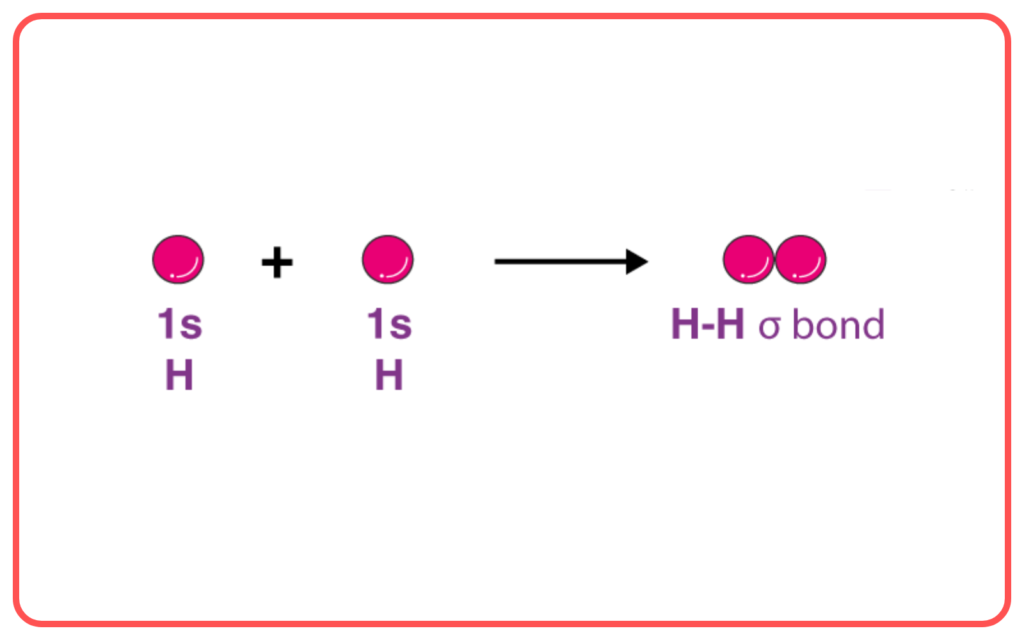
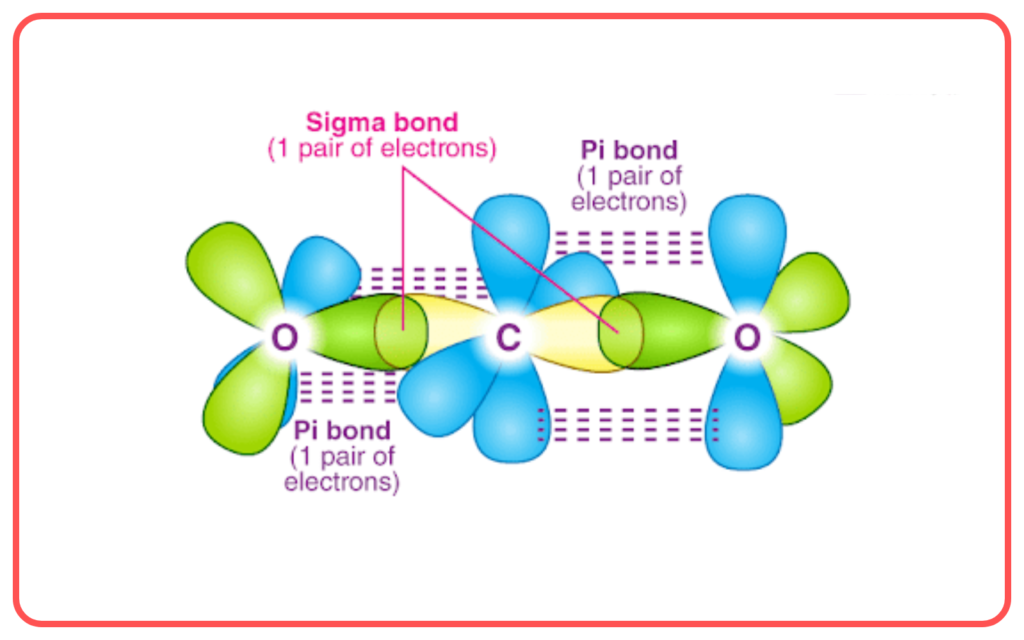
- Sigma (σ) Bond: A sigma bond is formed by the head-on overlap of atomic orbitals (e.g., s-s, s-p, or p-p orbitals). This type of bond is strong, allowing free rotation around the bond axis.
- Pi (π) Bond: A pi bond forms when p-orbitals overlap sideways. It is weaker than a sigma bond and restricts the rotation of the bonded atoms.
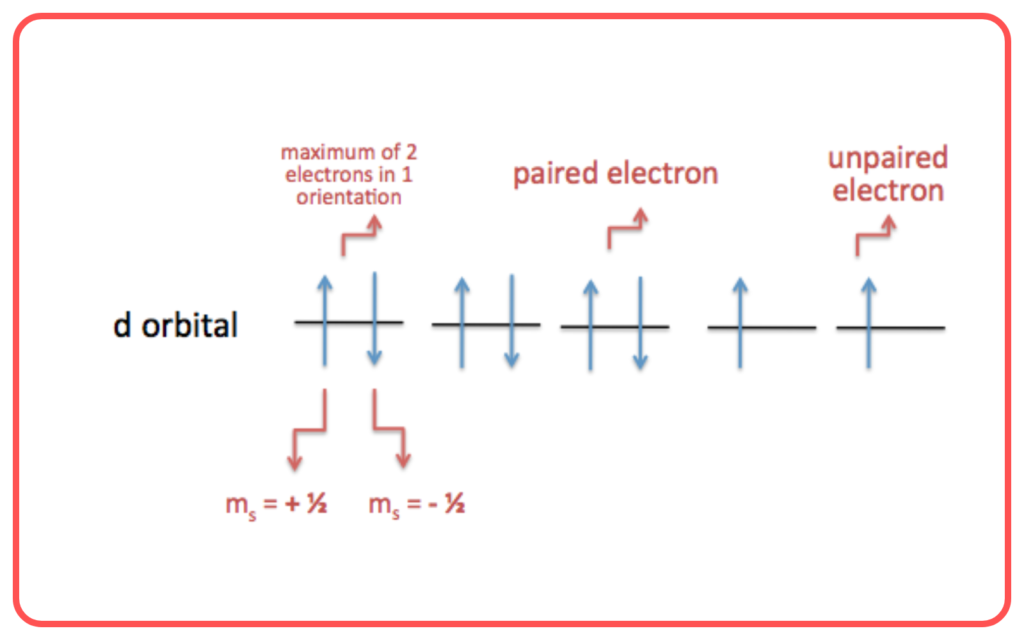
4. Spin Pairing
For a bond to form, the electrons in the overlapping orbitals must have opposite spins. This pairing of electrons stabilizes the bond.
5. Molecular Shapes
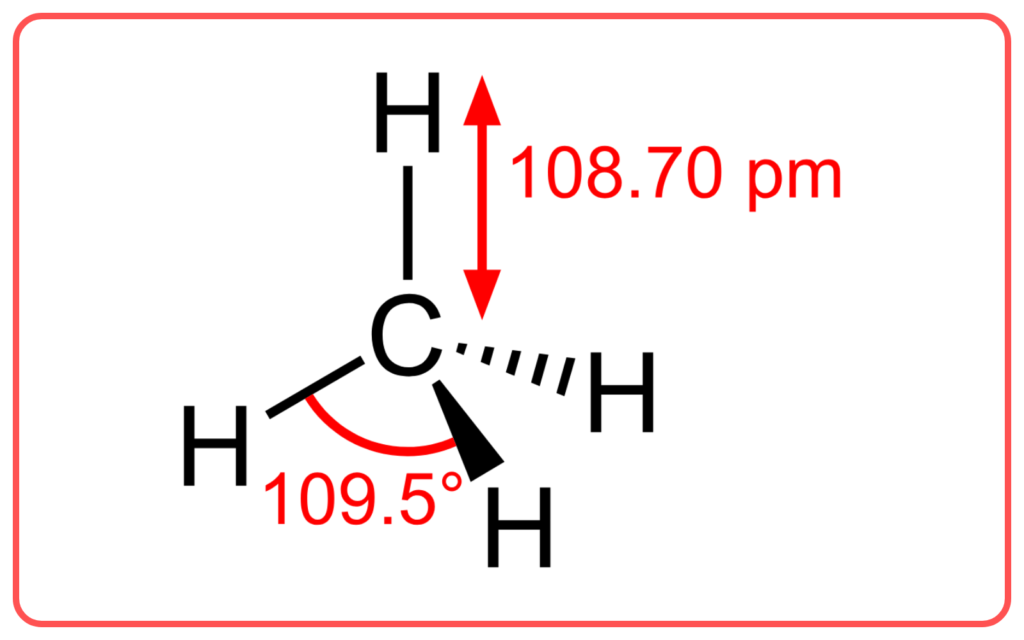
VBT explains the directionality of bonds. The way atomic orbitals overlap affects the geometry or shape of the molecule. For example, in methane (CH₄), the overlap of the sp³ hybridized orbitals leads to a tetrahedral shape.
6. Applications of VBT
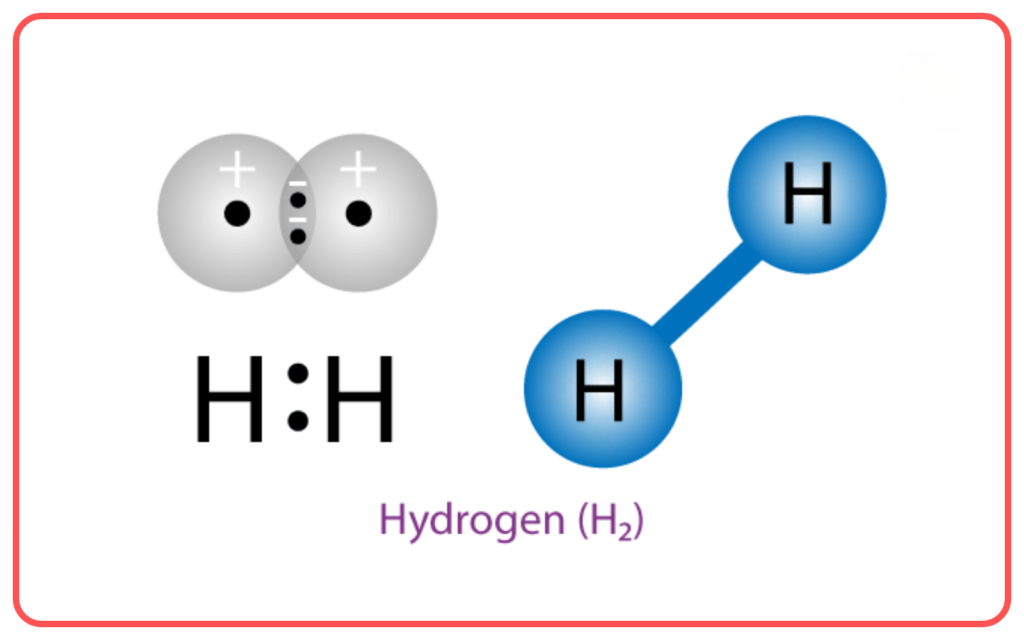
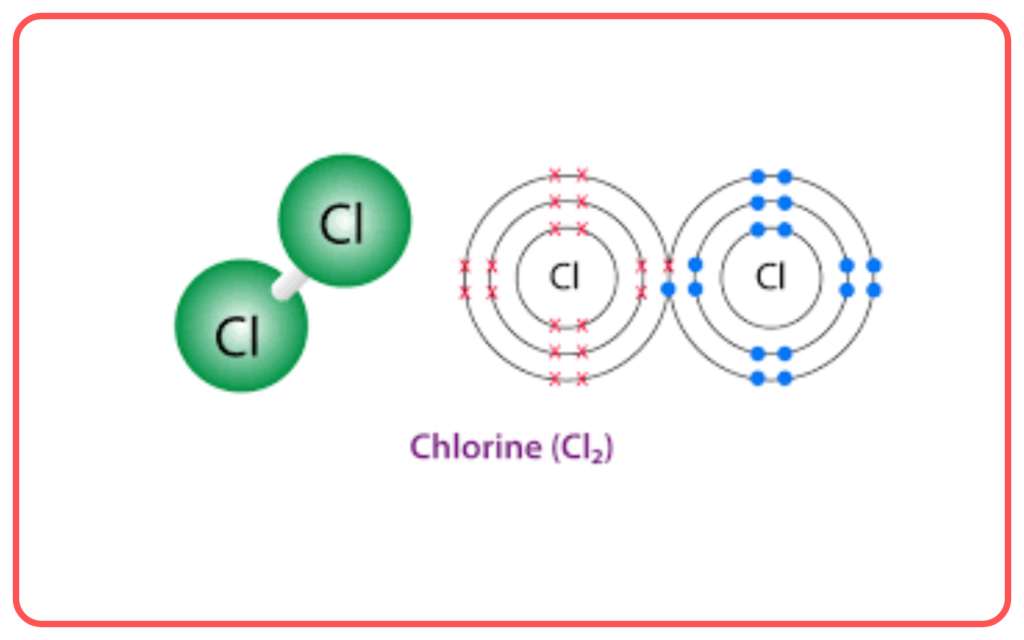
- VBT is effective in explaining the bonding and structure of simple molecules like H₂, Cl₂, and CH₄.
- It predicts bond strength and bond angles by considering the extent of orbital overlap.
- It also provides insight into how atomic orbitals combine to form stable molecules.
Limitations of VBT
- VBT does not explain magnetic properties of molecules, such as the paramagnetism of oxygen (O₂).
- It cannot account for resonance structures, like those in benzene (C₆H₆), where electrons are delocalized.
- VBT is less effective than Molecular Orbital Theory (MOT) in explaining the overall energy of a molecule and the distribution of electrons in molecular orbitals.
Let’s practice!

