Drawbacks of Electronic Theory Of Valence
Key Notes:
Shapes of Molecules
The theory does not explain the geometrical shapes of molecules. For instance, it cannot clarify why water (H₂O) has a bent shape or why methane (CH₄) forms a tetrahedral structure.
2. Bond Strength
It fails to explain differences in bond strength. For example, it cannot address why double bonds are stronger than single bonds but weaker than triple bonds
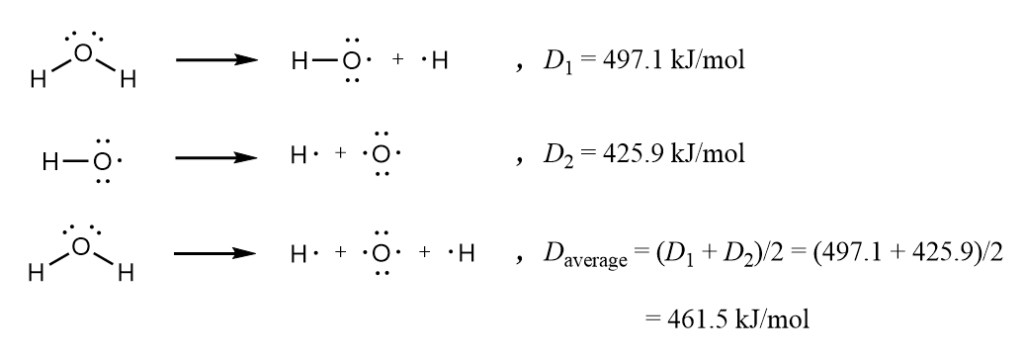
3. Transition Elements
The theory is inadequate for transition elements, which involve d-orbitals. It does not explain variable valency or metallic bonding observed in these elements.

4. Octet Rule Exceptions
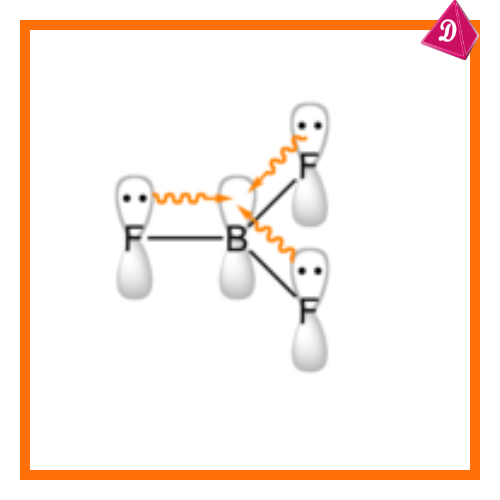
The theory overemphasizes the octet rule, ignoring molecules that violate it:
- Incomplete octet: Boron trifluoride (BF₃).
- Expanded octet: Sulfur hexafluoride (SF₆).
5. Neglect of Quantum Mechanics
It does not incorporate quantum mechanical principles such as hybridization, which explains molecular shapes, or molecular orbital theory, which addresses bonding in detail.
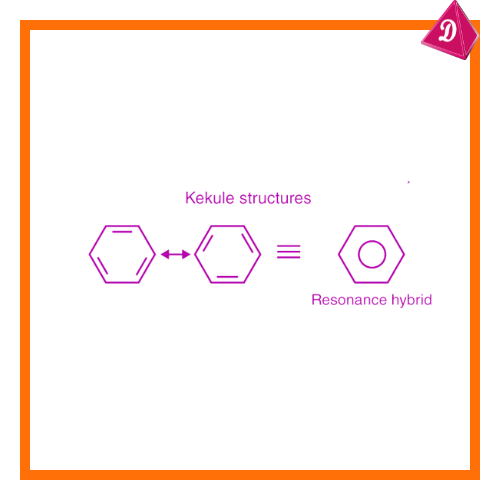
6. Resonance
The theory cannot explain resonance, where a molecule has multiple valid structures, such as benzene (C₆H₆) and ozone (O₃).
Let’s practice!

