Electronic Configuration
Electronic Configuration
Key Notes:
Introduction
- Electronic Configuration refers to the arrangement of electrons in the orbitals of an atom.
- It follows specific rules and principles that govern the distribution of electrons in different energy levels, sublevels, and orbitals.
Key Concepts
- Energy Levels (Shells):
- Represented by the Principal Quantum Number (nn).
- Examples: n=1,2,3,…
- Sublevels (Subshells):
- Each energy level has sublevels (s,p,d,f).
- Each sublevel has specific orbitals:
- s: 1 orbital (2 electrons).
- p: 3 orbitals (6 electrons).
- d: 5 orbitals (10 electrons).
- f: 7 orbitals (14 electrons).
- Orbitals:
- Regions around the nucleus where electrons are most likely found.
- Each orbital can hold 2 electrons with opposite spins.
Rules for Writing Electronic Configuration
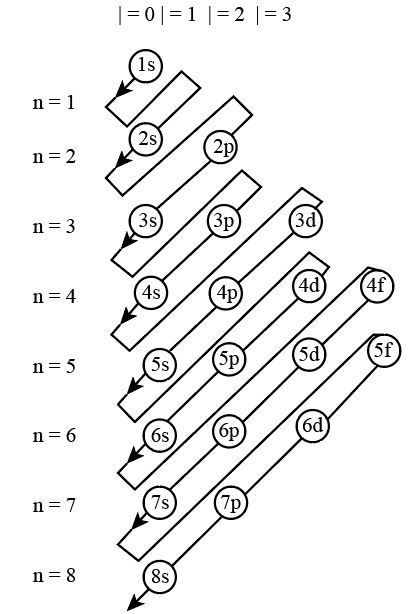
- Aufbau Principle:
- Electrons fill orbitals in order of increasing energy.
- Energy order: 1s<2s<2p<3s<3p<4s<3d<4p…
- Pauli Exclusion Principle:
- No two electrons in an atom can have the same set of all four quantum numbers.
- Each orbital can hold a maximum of 2 electrons with opposite spins.
- Hund’s Rule:
- Electrons occupy degenerate orbitals (orbitals of the same energy) singly first, before pairing.
Notation
- Standard Notation:
- Represented as nlⁿᵘᵐᵇᵉʳ ᵒᶠ ᵉˡᵉᶜᵗʳᵒⁿˢ.
- Example: 1s²2s² 2p⁶.
- Orbital Diagram:
- Uses arrows to show electron spins.
- Example:
1s ↑↓, 2s ↑↓, 2p ↑ ↑ ↑
Examples of Electronic Configurations
- Hydrogen (Z=1Z = 1): 1s¹.
- Helium (Z=2Z = 2): 1s².
- Carbon (Z=6Z = 6): 1s² 2s² 2p²s
- Neon (Z=10Z = 10): 1s²2s² 2p⁶
Electronic Configuration of Elements in the Periodic Table
- Groups: Elements in the same group have similar outermost configurations.
- Example: Group 1: ns¹.
- Periods: Elements in the same period have the same number of energy levels.
Shortcut Notation (Noble Gas Configuration)
- For elements with many electrons, configurations can be abbreviated using noble gases:
- Example: Sodium (Z=11): [Ne] 3s¹.
Importance of Electronic Configuration
- Chemical Properties:
- Determines reactivity, valency, and bonding.
- Periodic Trends:
- Explains trends like atomic size, ionization energy, and electronegativity.
- Stability:
- Atoms with fully filled or half-filled orbitals are more stable.
Let’s practice!

