Bohr-Somerfield Model Of An Atom
Key Notes:
Introduction
- Proposed by Arnold Sommerfeld in 1916 as an extension of Bohr’s Model of the atom.

- Addressed the limitations of Bohr’s model by introducing elliptical orbits in addition to circular ones.
- Explained fine structure (splitting) of spectral lines observed in atoms like hydrogen.
Key Features of Bohr-Sommerfeld Model
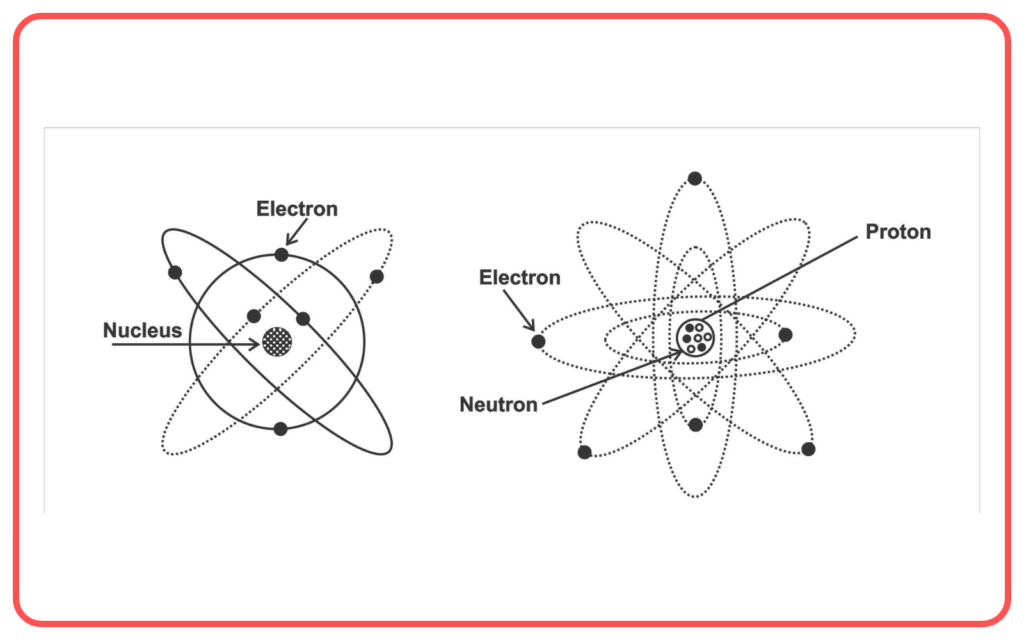
- Elliptical Orbits
- Electrons can move in both circular and elliptical orbits around the nucleus.
- The nucleus is located at one of the focal points of the ellipse.
- Two Quantum Numbers
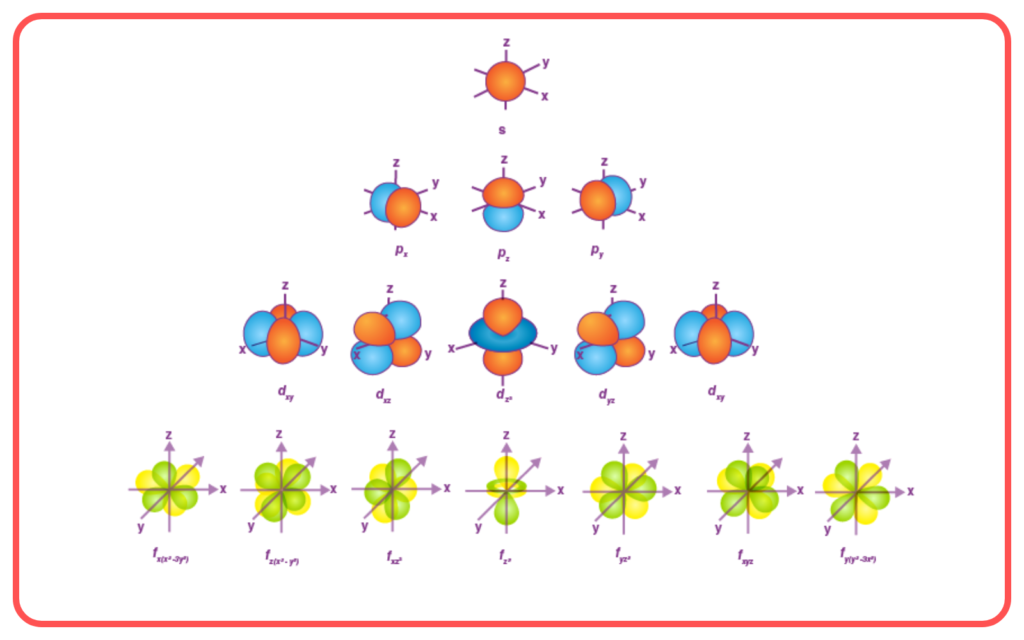
- Introduced two quantum numbers to describe electron motion:
- Principal Quantum Number (n): Determines the size and energy of the orbit.Azimuthal Quantum Number (k): Determines the shape of the orbit.
- k ranges from 1 to n and defines how elliptical the orbit is.
- Introduced two quantum numbers to describe electron motion:
- Orbital Energy and Angular Momentum
- The total energy of the electron depends on its principal quantum number (n), similar to Bohr’s model.
- The angular momentum of an electron in elliptical orbits is quantized: L=kh/2π,k≤n
- Relativistic Effects
- Sommerfeld introduced relativistic corrections for high-speed electrons in inner orbits, which improved the accuracy of predictions for spectral lines.
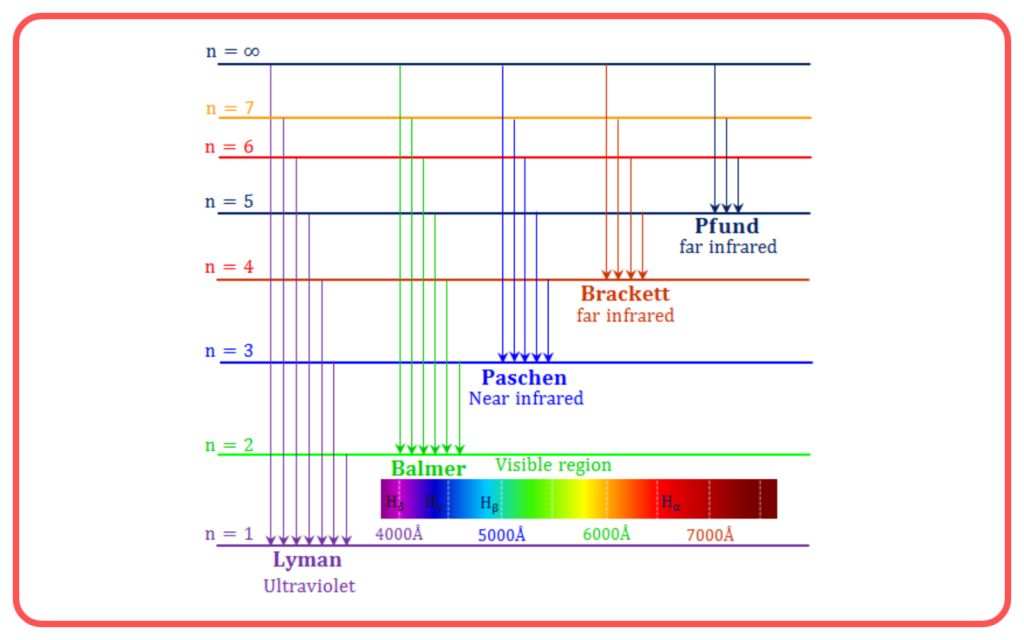
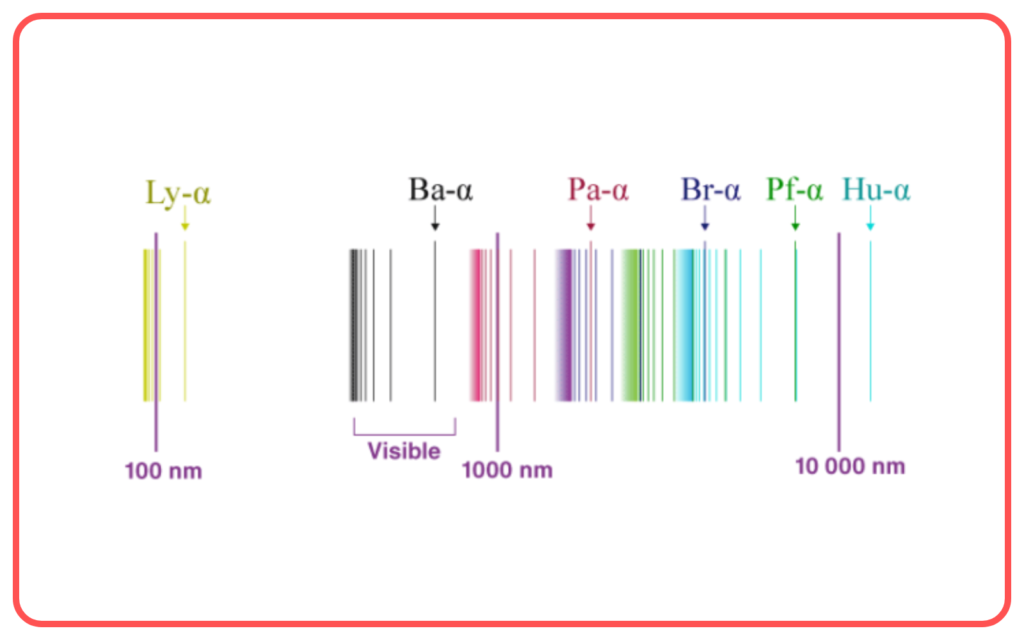
Fine Structure of Spectral Lines
- The model explained the splitting of spectral lines into closely spaced components due to the different shapes (elliptical and circular) of orbits.
- Provided a better understanding of the hydrogen spectrum compared to Bohr’s model.
Achievements of the Bohr-Sommerfeld Model
- Extended Bohr’s theory to explain fine structure in spectral lines.
- Incorporated elliptical orbits, making the model more realistic.
- Introduced additional quantum numbers, paving the way for quantum mechanics.
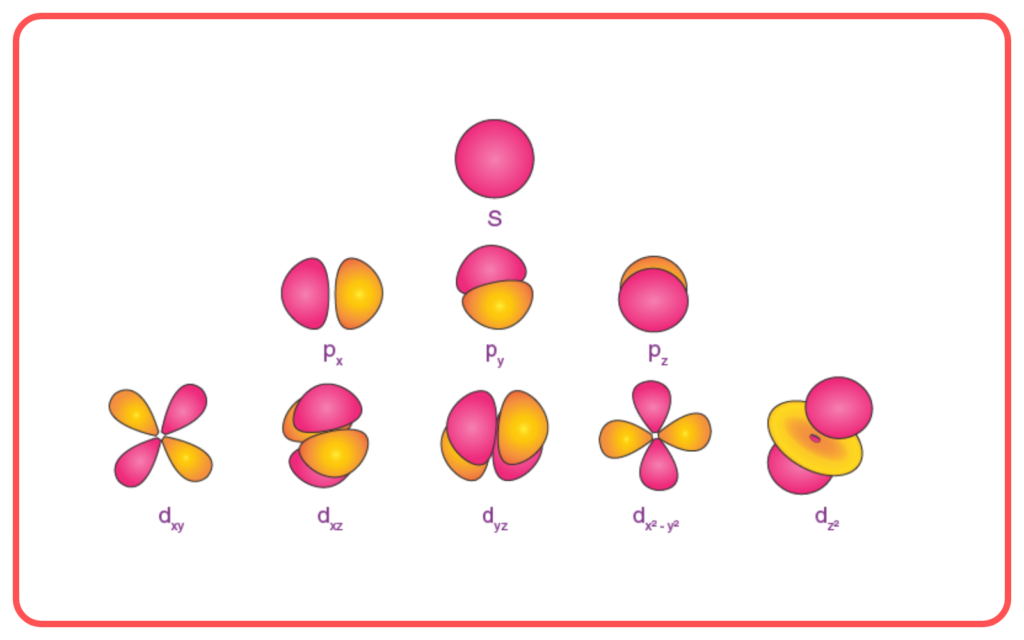
Limitations of the Bohr-Sommerfeld Model
- Applicable Only to Hydrogen-like Atoms
- Could not accurately explain spectra of multi-electron atoms.
- Failed to Explain Zeeman Effect
- Could not completely explain the splitting of spectral lines in magnetic fields.
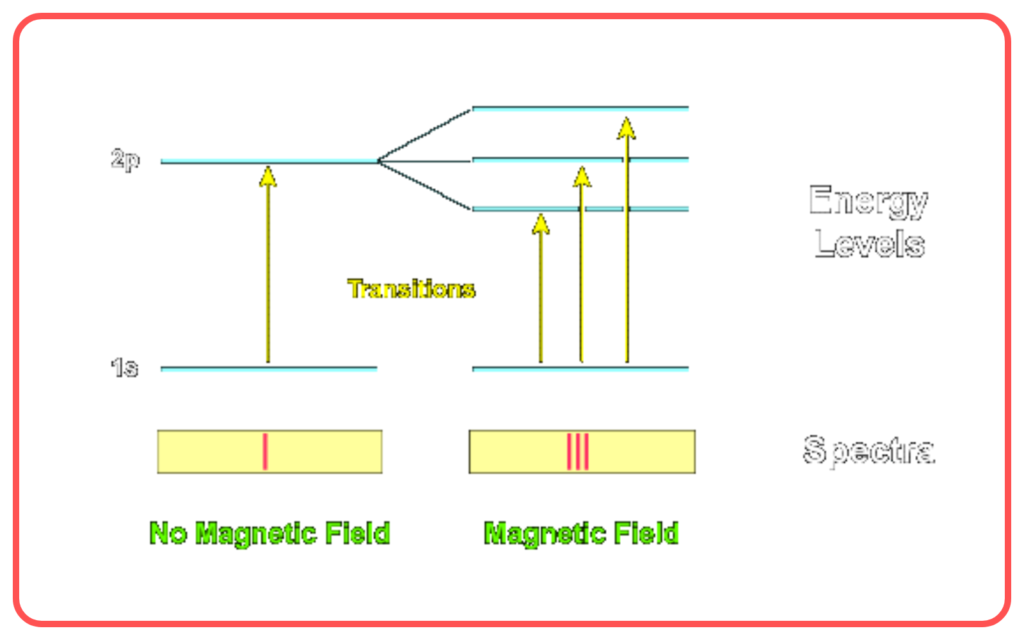
- Incompatibility with Wave-Particle Duality
- Did not incorporate the wave-like behavior of electrons (proposed later by de Broglie).
- Violation of Heisenberg’s Uncertainty Principle
- Assumed well-defined paths for electrons, which contradicts quantum mechanics.
- Superseded by Quantum Mechanical Model
- Could not explain finer details of atomic structure, which were addressed by the Schrödinger wave equation.
Comparison with Bohr’s Model
| Feature | Bohr’s Model | Bohr-Sommerfeld Model |
|---|---|---|
| Shape of Orbits | Circular only | Circular and elliptical |
| Quantum Numbers | Principal (n) only | Principal (n) and azimuthal (k) |
| Spectral Line Explanation | Broad hydrogen spectrum | Fine structure of hydrogen spectrum |
| Relativity | Not included | Included |
Conclusion
- The Bohr-Sommerfeld Model was an important step in refining atomic theory and bridging the gap between Bohr’s atomic model and the modern quantum mechanical model.
- It introduced critical concepts like elliptical orbits and quantum numbers that are foundational in understanding atomic behavior.
Let’s practice

