Electromagnetic Spectrum.
Key Notes:
Definition of Electromagnetic Spectrum
The electromagnetic spectrum refers to the range of all types of electromagnetic radiation, arranged according to their wavelength or frequency.
Electromagnetic Waves
- What are Electromagnetic Waves?
- Oscillating electric and magnetic fields perpendicular to each other and the direction of wave propagation.
- Travel at the speed of light (c=3×10⁸ m/s) in a vacuum.
- Key Characteristics:
- Wavelength (λ): Distance between two peaks (measured in meters).
- Frequency (f): Number of wave cycles per second (measured in Hertz).
- Relationship: c=λf, where c is the speed of light.
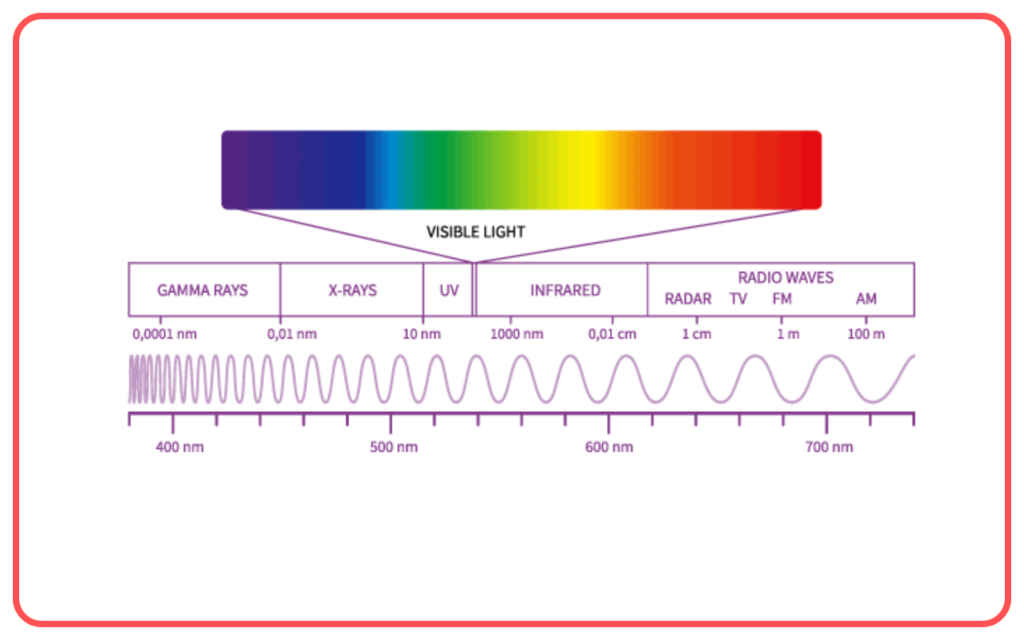
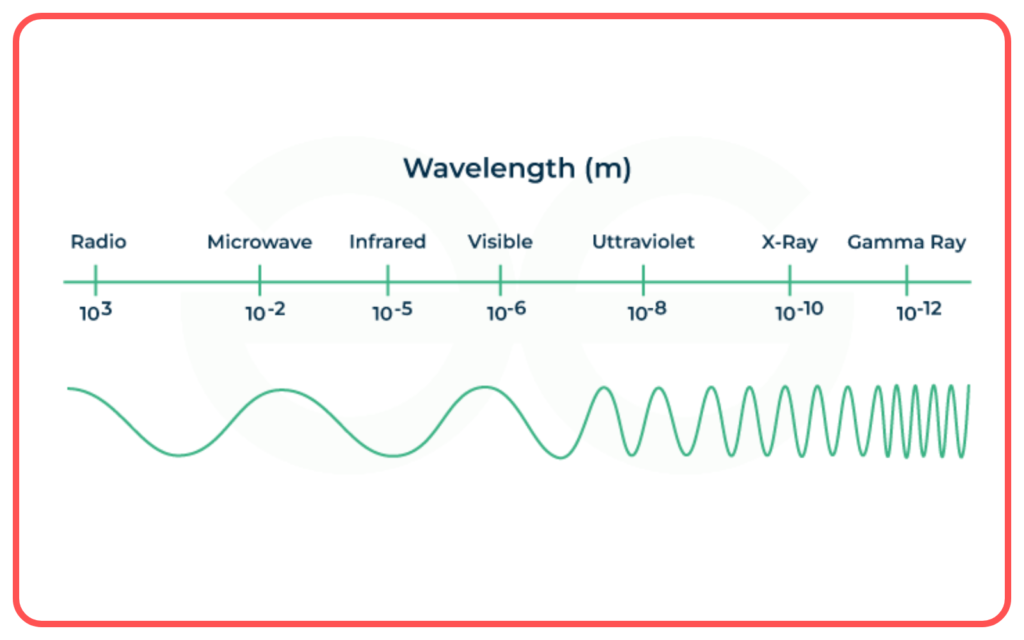
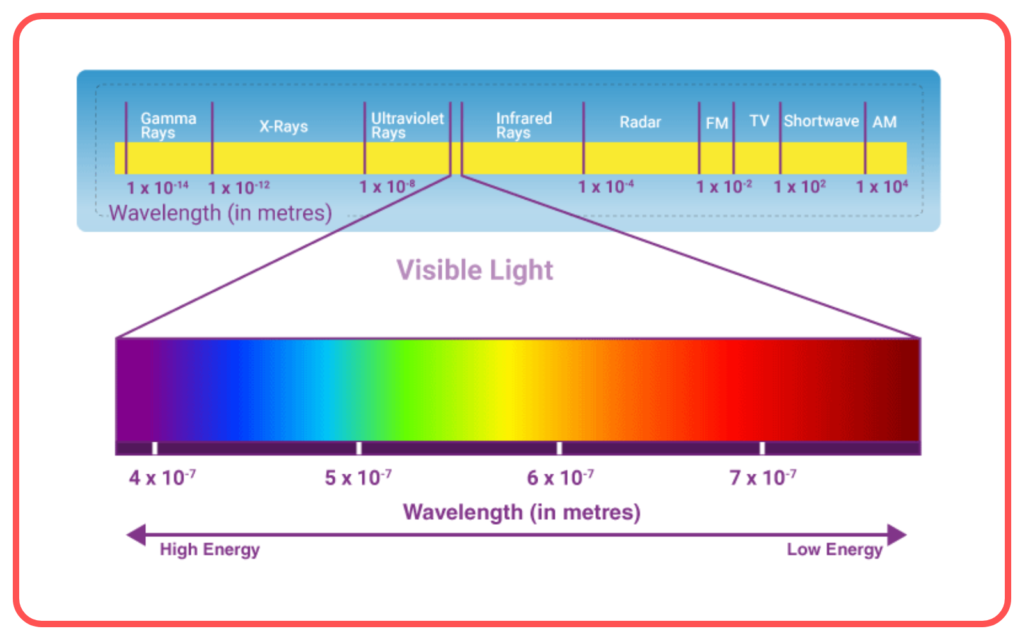
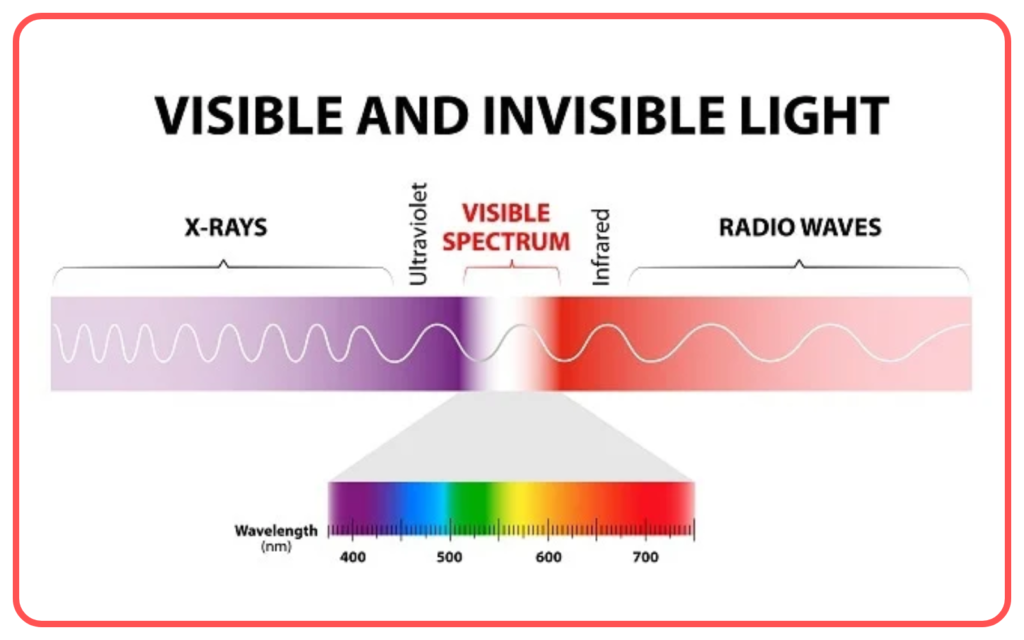
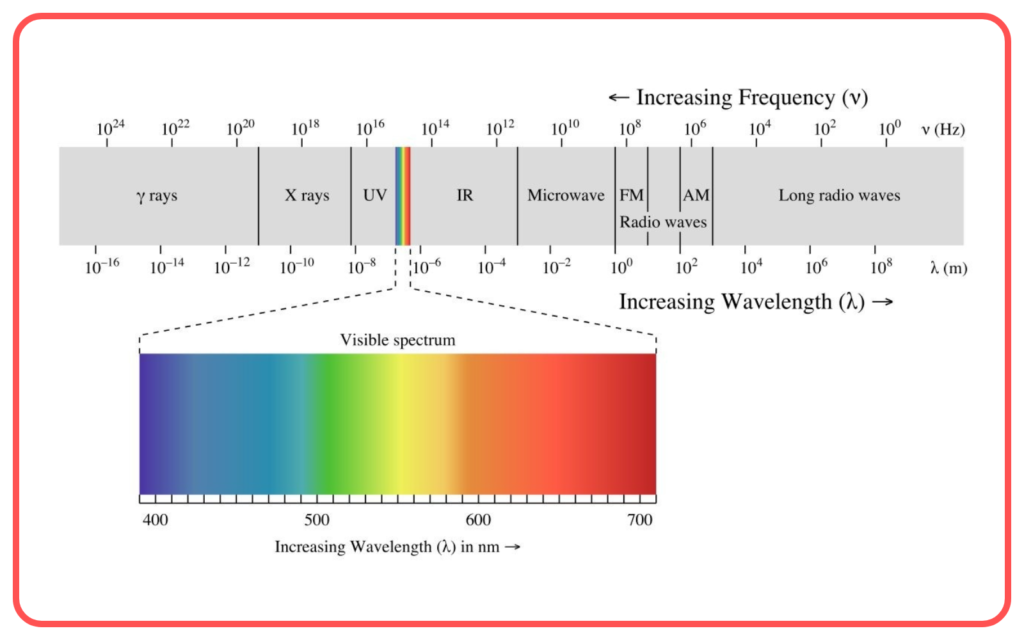
Components of Electromagnetic Spectrum
The electromagnetic spectrum includes the following regions, arranged by increasing frequency and decreasing wavelength:
- Radio Waves
- Longest wavelength, lowest frequency.
- Applications: Broadcasting (TV, radio), mobile communication.
- Microwaves
- Used in cooking, satellite communication, and radar systems.

- Infrared (IR) Radiation
- Heat radiation.
- Applications: Remote controls, thermal imaging, and weather forecasting.

- Visible Light
- The only part of the spectrum visible to the human eye.
- Colors range from violet (shortest wavelength) to red (longest wavelength).
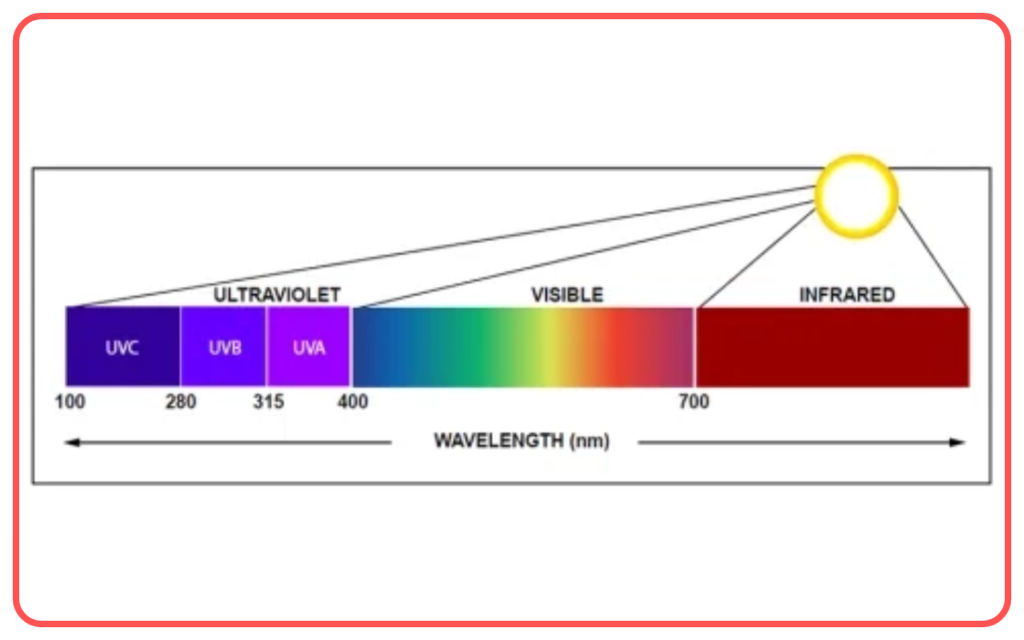
- Ultraviolet (UV) Radiation
- Higher energy than visible light.
- Applications: Sterilization, detection of counterfeit currency.
- Overexposure can cause skin damage.

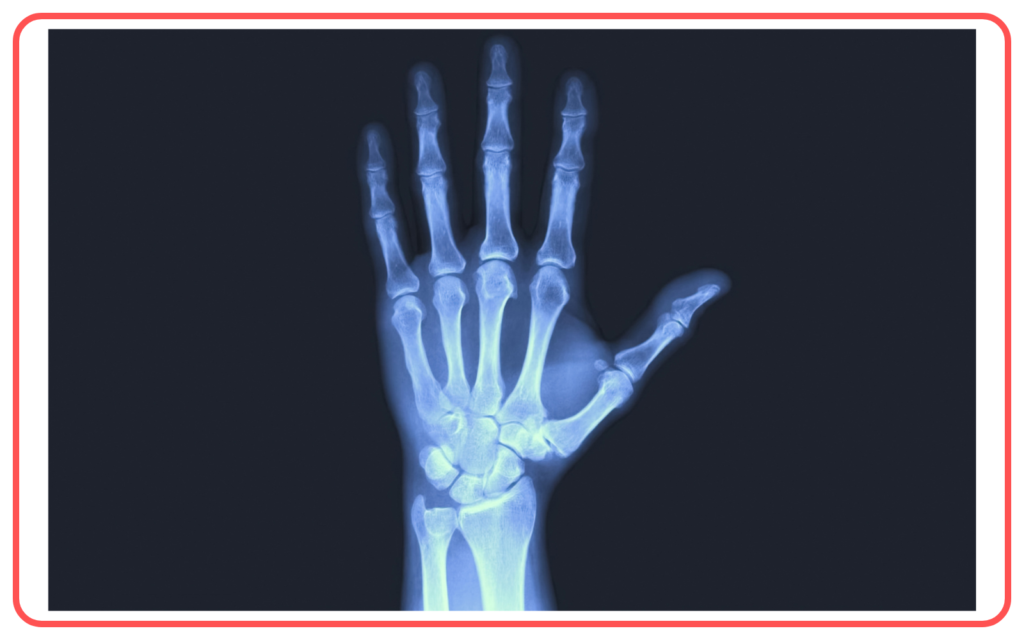
- X-Rays
- High energy and penetrative power.
- Applications: Medical imaging and security scanning.
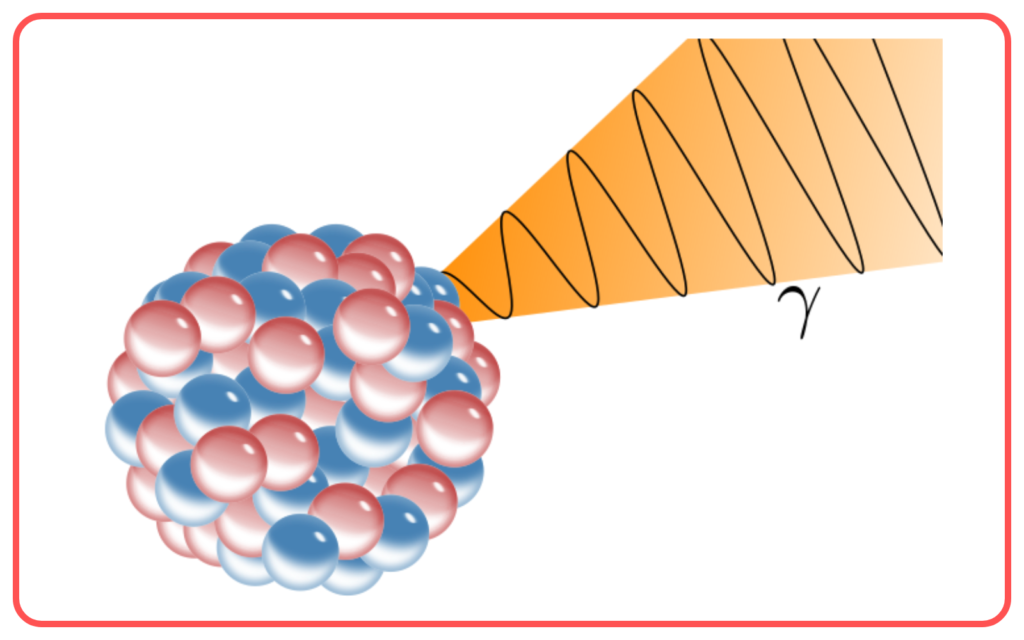
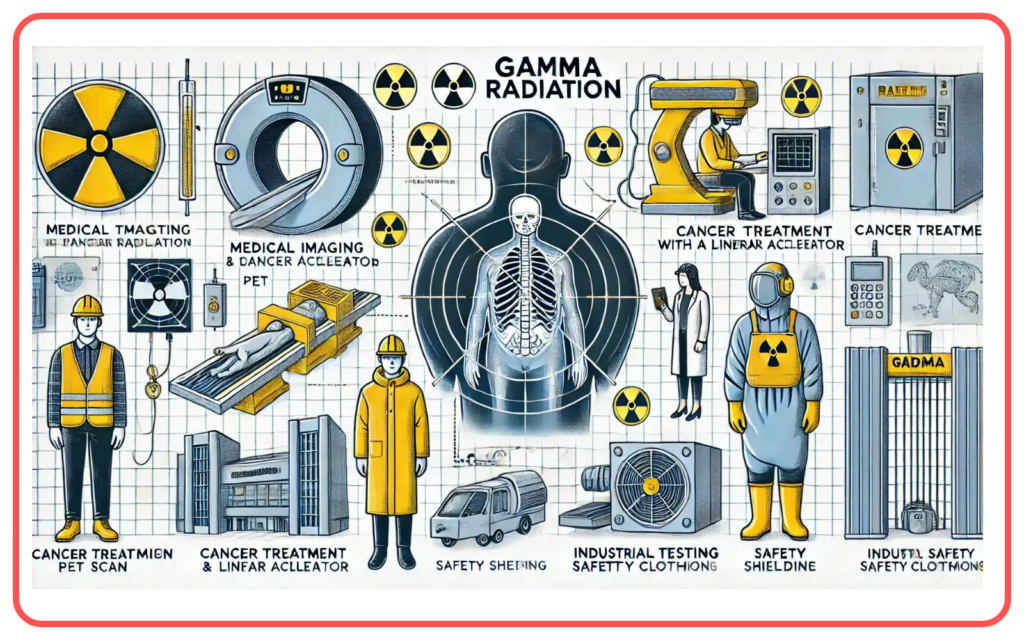
- Gamma Rays
- Shortest wavelength, highest energy.
- Applications: Cancer treatment, nuclear reactions.
Order of the Electromagnetic Spectrum
Increasing Frequency (Decreasing Wavelength):
Radio waves → Microwaves → Infrared → Visible Light → Ultraviolet → X-rays → Gamma rays
Wave Properties Across the Spectrum
- Radio Waves: Wavelengths longer than 1 mm, low frequency.
- Gamma Rays: Wavelengths shorter than 10⁻¹²m, extremely high frequency.
Key Relationships
- Energy and Frequency:
E=hf, where h is Planck’s constant(6.626 x 10 ⁻³⁴ J/s)- Higher frequency = Higher energy.
- Wave Equation:
c=λf.
Applications of Electromagnetic Spectrum
- Communication: Radio and microwaves for TV, radio, and mobile networks.

- Medicine: X-rays and gamma rays for diagnostics and cancer therapy.
- Astronomy: Observing distant celestial objects using different parts of the spectrum.
- Daily Life: Infrared for heat sensing, UV in sunlight for Vitamin D production.

Significance of Electromagnetic Spectrum
- Explains the diverse behavior of waves in different regions.
- Provides a foundation for technologies like satellite communication, medical imaging, and scientific research.

Safety Precautions
- Overexposure to UV, X-rays, and gamma rays can be harmful.
- Use protective equipment like lead shields for X-ray exposure.
Let’s practice!

