Freezing
Key Notes:
Definition of Freezing
- Freezing is the process in which a liquid changes into a solid when it is cooled and energy is removed.
- It occurs when the temperature of the liquid decreases, causing its molecules to lose kinetic energy and move closer together, forming a solid.
Freezing Point
- The freezing point is the temperature at which a liquid turns into a solid at constant pressure.

- For pure water, the freezing point is 0°C or 273 K.
- The freezing point is the same as the melting point, but the direction of heat flow is reversed. While melting requires the absorption of heat, freezing releases heat.
Latent Heat of Freezing
- During freezing, the latent heat of freezing is released. This is the amount of heat energy released when a substance changes from a liquid to a solid at its freezing point.
- For water, the latent heat of freezing is the same as the latent heat of fusion, which is 334 J/g or 334,000 J/kg.
- This heat is released into the surroundings as the liquid forms a solid.
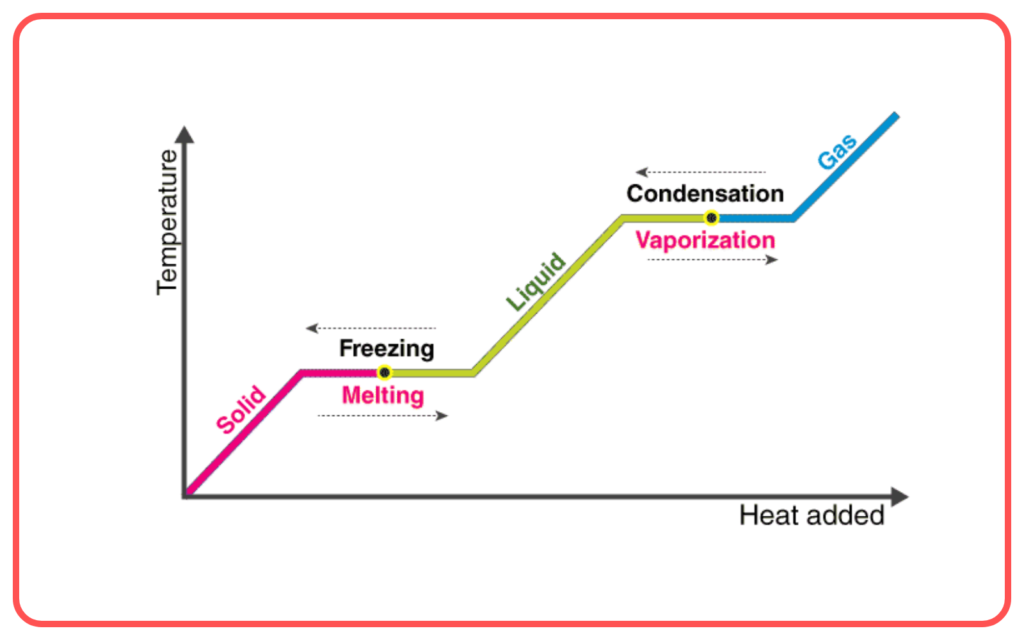
Factors Affecting Freezing
- Temperature: A liquid will freeze at its freezing point when the surrounding temperature drops below this threshold.
- Pressure: For most substances, the freezing point decreases with an increase in pressure. For example, the freezing point of water decreases at higher altitudes where atmospheric pressure is lower. In contrast, increasing the pressure can increase the freezing point for some substances.

- Impurities: The presence of impurities in a liquid can lower its freezing point. For example, adding salt to water lowers its freezing point (this is why salt is used to melt ice on roads during winter).
Examples of Freezing
- Water to Ice: Water freezes into ice when the temperature drops to 0°C. This is the most common example of freezing.
- Liquid Food Products: Food items like juice, milk, and meat freeze in refrigerators or freezers to preserve them.

- Formation of Ice Cubes: When water in a tray is placed in a freezer, it freezes into ice cubes as the temperature drops below 0°C.

- Freezing of Lava: When molten lava from a volcano cools and solidifies, it freezes into solid rock.

Freezing in Nature
- Formation of Ice: In cold climates, water bodies like lakes, rivers, and ponds freeze when the air temperature drops below 0°C. This allows ice to form on the surface, creating ice sheets.

- Winter Weather: Precipitation such as rain or snow can freeze when temperatures drop, causing freezing rain or the formation of snow and ice.

- Permafrost: In polar regions, the ground remains frozen year-round, forming permafrost, a layer of soil or rock that is permanently frozen.

Freezing vs. Melting
- Freezing is the process where a liquid turns into a solid.
- Melting is the opposite of freezing; it is the process where a solid turns into a liquid.
- Melting absorbs heat (endothermic), while freezing releases heat (exothermic).

Applications of Freezing
- Food Preservation: Freezing is widely used to preserve food by slowing down the growth of bacteria and preventing spoilage. Foods like meat, vegetables, and fruits are frozen to extend their shelf life.

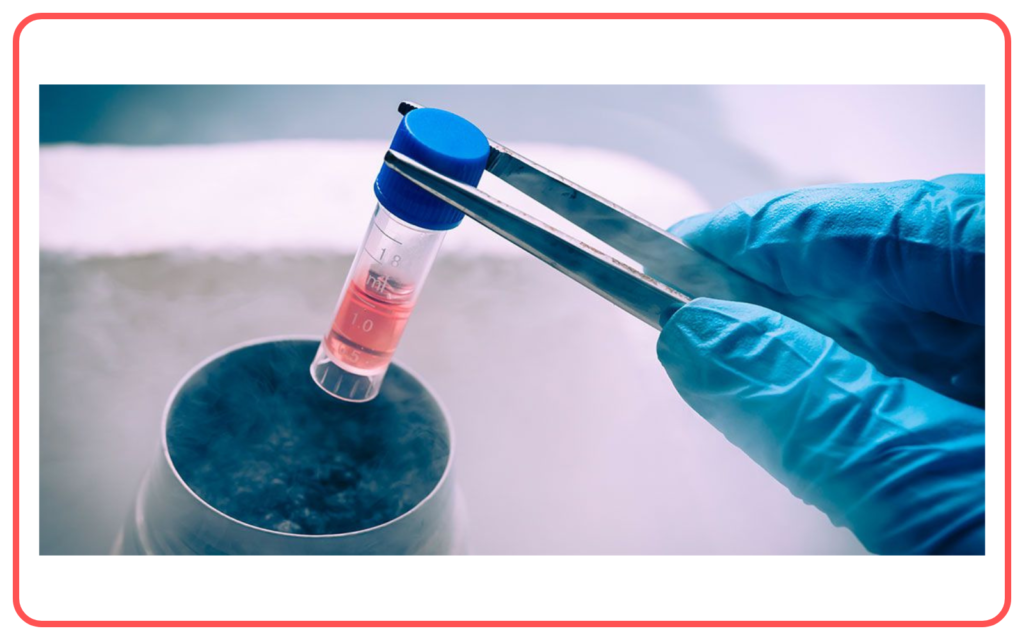
- Cryopreservation: Freezing is used in biological preservation, such as in the preservation of sperm, eggs, and other biological tissues for medical purposes.
- Refrigeration: In everyday life, freezers and refrigerators use freezing to store food and beverages at low temperatures to keep them fresh.
- Weather and Climate: Freezing plays an important role in the Earth’s climate system, including the formation of ice caps, glaciers, and snow cover, which are crucial for regulating the Earth’s temperature.

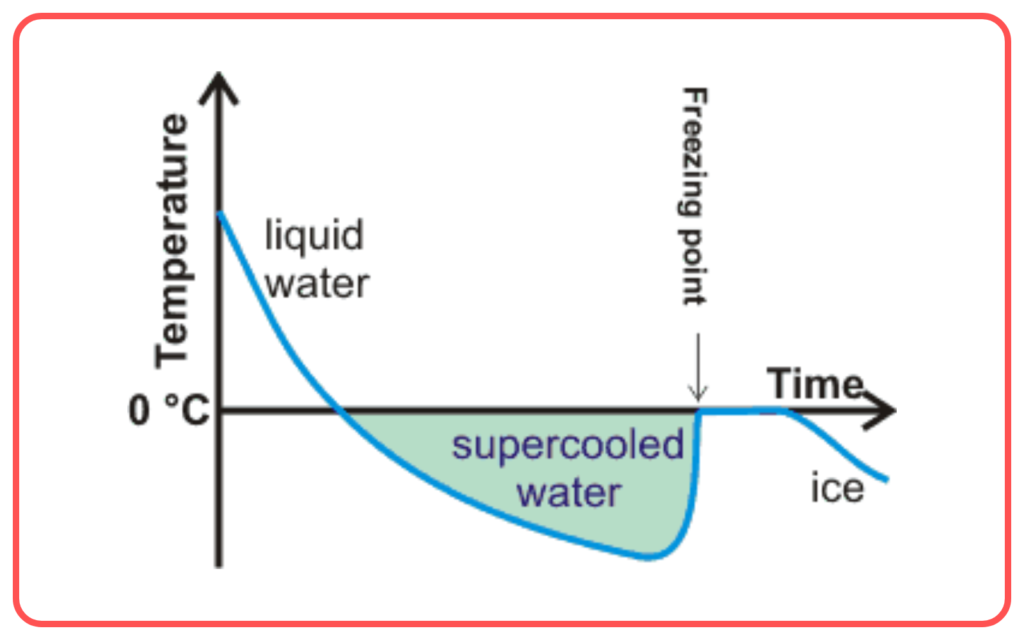
Supercooling
- Supercooling occurs when a liquid is cooled below its freezing point without actually freezing. This happens because the liquid has not formed any ice crystals yet. If disturbed, it can freeze suddenly and rapidly.
- Example: Water can sometimes be cooled below 0°C without freezing if it is free of impurities and air bubbles, but it will freeze instantly when disturbed.
Freezing and Environmental Impact
- Frozen Water Resources: The freezing and melting of ice play an important role in maintaining freshwater resources. In polar regions, the melting of glaciers and ice caps can contribute to rising sea levels.

- Climate Change: As global temperatures rise, the process of freezing (and the stability of ice) is affected. This leads to the melting of glaciers and polar ice, impacting ecosystems and human populations.

Conclusion
- Freezing is a crucial physical process in which a liquid becomes a solid by losing heat.
- It is the opposite of melting and plays an essential role in nature, food preservation, and even in medical applications like cryopreservation.
- Understanding freezing helps explain natural phenomena, including the formation of ice and snow, and has significant applications in everyday life and various industries.
Let’s practice!

