Melting
Key Notes:
Definition of Melting
- Melting is the process in which a solid changes into a liquid when it is heated or when energy is supplied to it.

- The solid absorbs heat energy, which increases the kinetic energy of the particles. As a result, the particles move more freely and break away from their fixed positions, transforming the solid into a liquid.
Melting Point
- The melting point is the temperature at which a solid turns into a liquid at constant pressure.

- Different substances have different melting points. For example:
- Ice (solid water) melts at 0°C (273 K).
- Iron melts at 1538°C (1811 K).
- Gold melts at 1064°C (1337 K).
- The melting point depends on the type of material and the strength of the forces between its particles (atoms, molecules, or ions).
Latent Heat of Fusion
- During the process of melting, the solid absorbs latent heat (heat energy) without an increase in temperature. This energy is called latent heat of fusion.
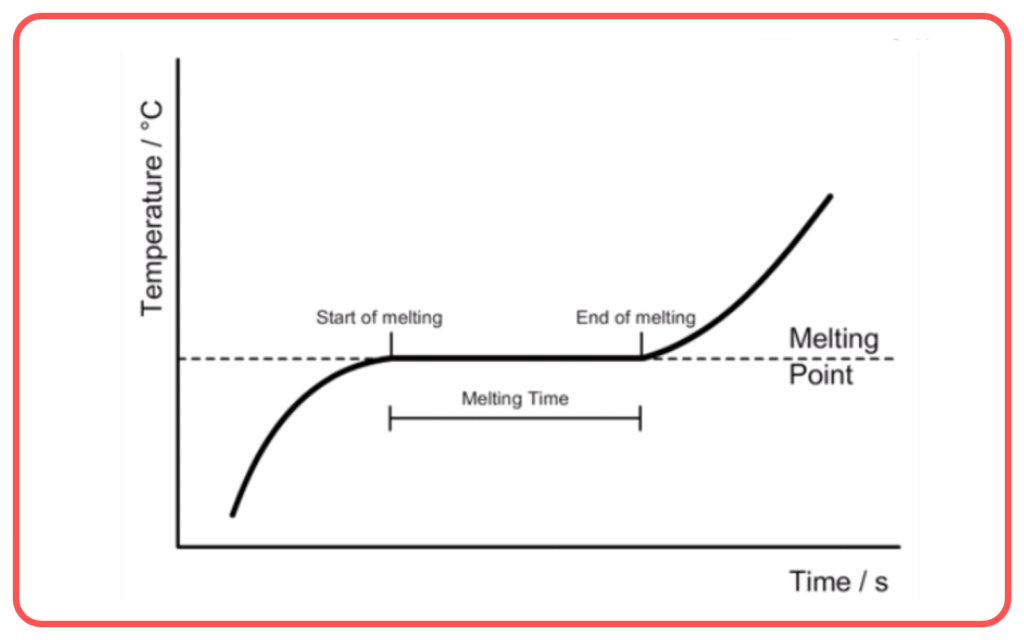
- Latent heat of fusion is the amount of heat required to change 1 kg of a solid into a liquid at its melting point.
- For water, the latent heat of fusion is 334 J/g or 334,000 J/kg.
- The heat absorbed is used to overcome the forces holding the particles together in the solid state.
Conditions for Melting
- Temperature: To melt a substance, its temperature must be raised to its melting point.

- Pressure: For most solids, melting occurs at a specific temperature under standard pressure. However, for some substances like ice, the melting point can decrease if pressure is increased. For instance, pressure causes ice to melt at temperatures below 0°C at higher altitudes.
Examples of Melting
- Ice melts into water when the temperature reaches 0°C.

- Butter melts when heated from its solid form at room temperature into a liquid state.

- Chocolate melts when it is exposed to heat and turns into a liquid.

- Metals like copper and gold melt when heated to high temperatures.

Factors Affecting Melting
- Nature of the substance: Different substances melt at different temperatures depending on the strength of the forces between the particles. For example, ionic compounds like salt have high melting points due to strong ionic bonds.
- Pressure: As pressure increases, the melting point of some substances (like water) decreases. Conversely, for other materials (like metals), higher pressure might increase the melting point.
- Purity of the substance: Impurities in a substance can lower or raise its melting point. For example, adding salt to ice lowers its melting point, a phenomenon used in de-icing roads.
Melting in Daily Life
- Cooking: The process of melting is often seen when cooking. For example, butter melts when added to a hot pan.

- Glaciers and Icebergs: Ice melts when the temperature increases, contributing to the melting of glaciers and icebergs in warm weather.

- Ice Cream: Ice cream melts when exposed to heat, turning from a solid to a creamy liquid.

- Candle Wax: When a candle is lit, the heat from the flame causes the wax to melt into liquid form, which then solidifies when cooled.

Melting in Nature
- Formation of Water: Water in solid form (ice) melts into liquid form in warm weather or when heat is supplied. This is part of the water cycle, where ice and snow in the environment melt and become liquid water that can be used in the ecosystem.
- Glaciers: The melting of glaciers due to global warming is a significant environmental issue, as it contributes to rising sea levels.

Melting vs. Freezing
- Melting is the process where a solid turns into a liquid.

- Freezing is the opposite of melting; it is the process where a liquid turns into a solid.
- Melting absorbs heat (endothermic process), while freezing releases heat (exothermic process).

Applications of Melting
- Metal Casting: Metals like iron, aluminum, and gold are melted and poured into molds to create various products, such as tools, machinery parts, and jewelry.

- Snow and Ice Removal: Melting salt is often used in colder regions to help melt ice on roads and prevent accidents during winter.
- Food Preparation: In the food industry, melting is used in cooking processes such as making sauces, melting butter or cheese, and in the preparation of confections like chocolate.

Conclusion
- Melting is a fundamental process in physics and chemistry where a solid turns into a liquid when heat is added.

- It plays an essential role in various natural and industrial processes, from the formation of liquid water in the water cycle to the melting of metals for manufacturing.
- Understanding melting is crucial for applications in daily life, industry, and environmental studies.
Let’s practice!

