Specific Heat
Key Notes:
Definition of Specific Heat
- Specific heat (also called specific heat capacity) is the amount of heat energy required to raise the temperature of 1 kilogram of a substance by 1°C (or 1 K).
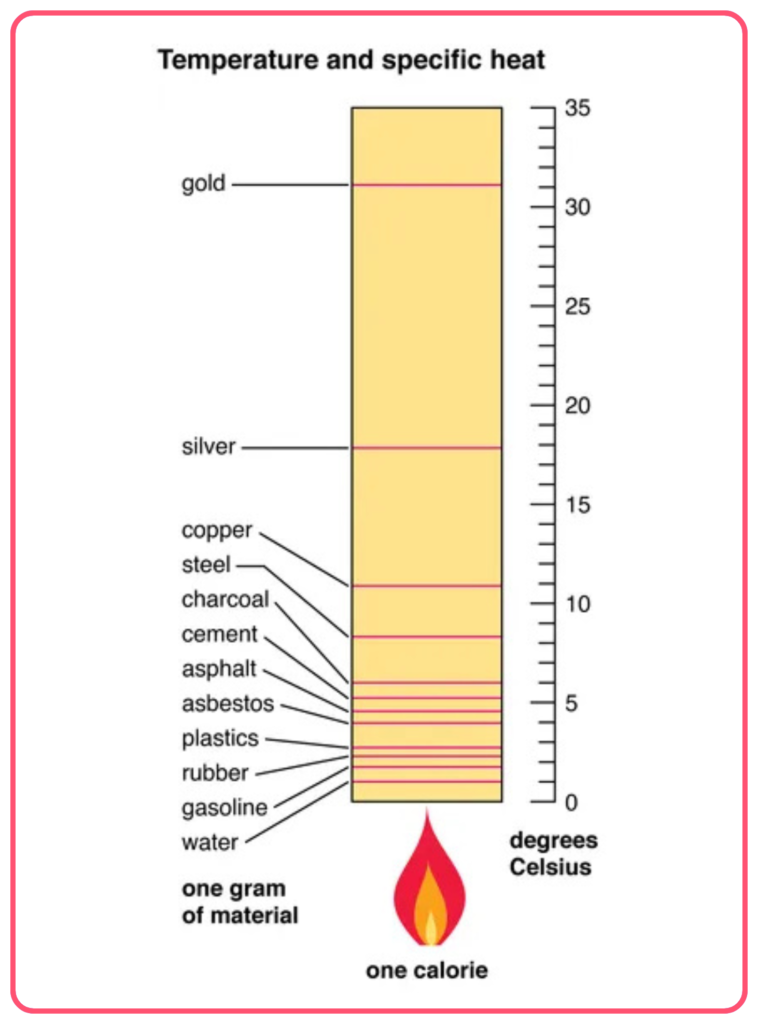
- It is a material property and varies from substance to substance.
Formula for Specific Heat
The formula to calculate the specific heat is: Q=mcΔT
Where:
- Q = Heat energy absorbed or released (in joules, J)
- m = Mass of the substance (in kilograms, kg)
- c = Specific heat capacity of the substance (in joules per kilogram per degree Celsius, J/kg°C)
- ΔT = Change in temperature (in degrees Celsius or Kelvin)
Units of Specific Heat
- The SI unit of specific heat is Joules per kilogram per degree Celsius (J/kg°C).
- Sometimes, it is expressed as Joules per kilogram per Kelvin (J/kg K) since the temperature difference in Celsius and Kelvin is the same.
Factors Affecting Specific Heat
- Material type: Different substances have different specific heat capacities. For example, water has a high specific heat, meaning it requires a lot of heat energy to raise its temperature.
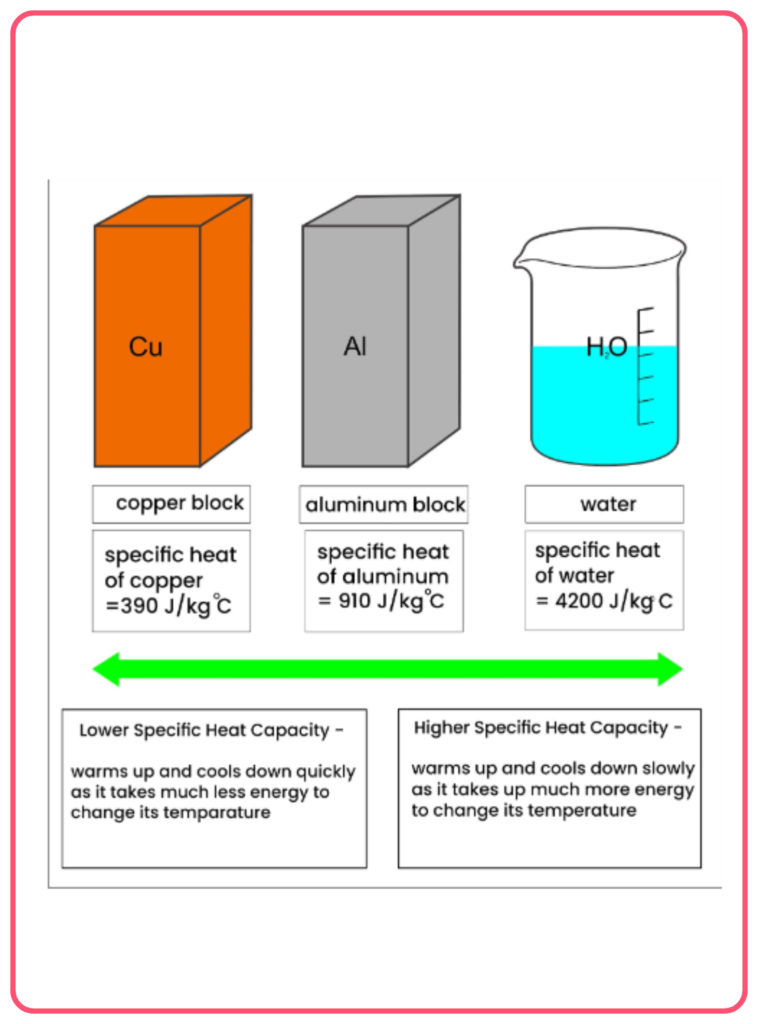
- Temperature: For most substances, the specific heat remains constant within a limited temperature range. However, it may vary slightly at very high or low temperatures.
Importance of Specific Heat
- Water’s high specific heat: Water has a relatively high specific heat capacity, meaning it can absorb a large amount of heat without a significant rise in temperature. This property is essential in regulating the Earth’s climate and in biological processes like temperature regulation in living organisms.
- Thermal regulation: Substances with high specific heat can act as heat reservoirs, absorbing and releasing heat slowly, which is important for maintaining stable temperatures in various applications (e.g., water in oceans or lakes).

Specific Heat of Different Substances
- Water: The specific heat of water is relatively high, about 4.18 J/g°C or 4180 J/kg°C. This makes water excellent at regulating temperature.
- Metals: Most metals have a low specific heat compared to water. For example:
- Iron: ~0.45 J/g°C
- Aluminum: ~0.90 J/g°C
- Copper: ~0.39 J/g°C
- Other materials: Gases like air and solid materials like wood also have lower specific heats compared to water.
Applications of Specific Heat
- Climate control: The high specific heat of water helps to moderate the Earth’s climate, ensuring that temperature changes do not occur too quickly.

- Cooling systems: Specific heat is critical in designing cooling systems (e.g., radiators, air conditioning), as materials with higher specific heats are better at absorbing and releasing heat.

- Cooking: The concept of specific heat explains why some materials, like water, heat up slowly compared to materials like metals, which heat up more quickly.

let’s practice!

