Evaporation
Key Notes:
Definition of Evaporation
- Evaporation is the process by which a liquid turns into a gas at the surface, even below its boiling point.
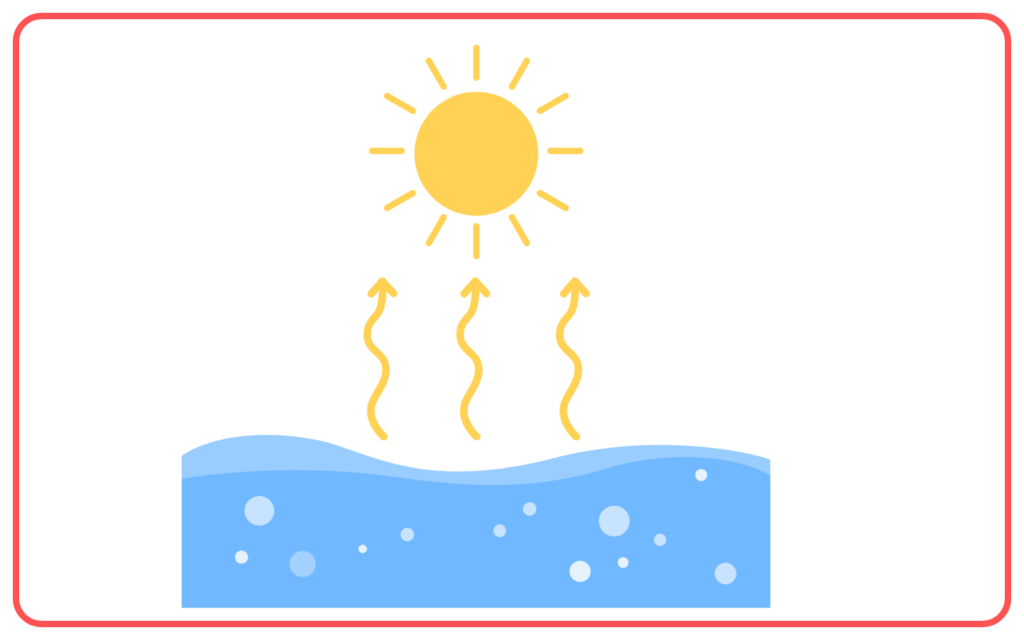
- It occurs at the surface of the liquid where molecules with enough energy escape into the air as vapor.
Characteristics of Evaporation
- Surface Phenomenon: Evaporation occurs only at the surface of the liquid.
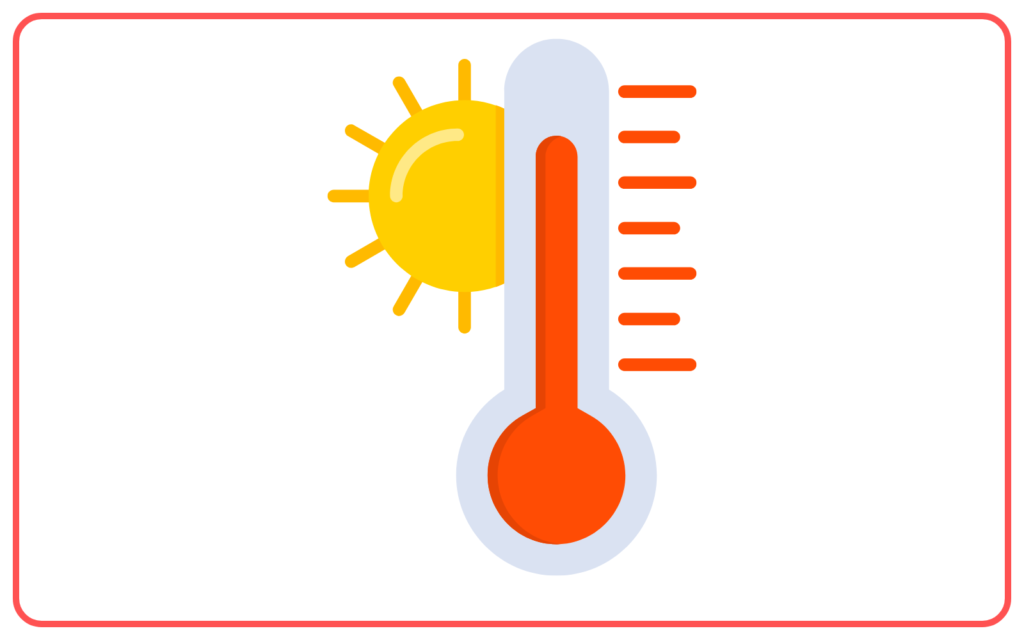
- Temperature Dependence: It happens at any temperature (not just at the boiling point), but faster at higher temperatures.
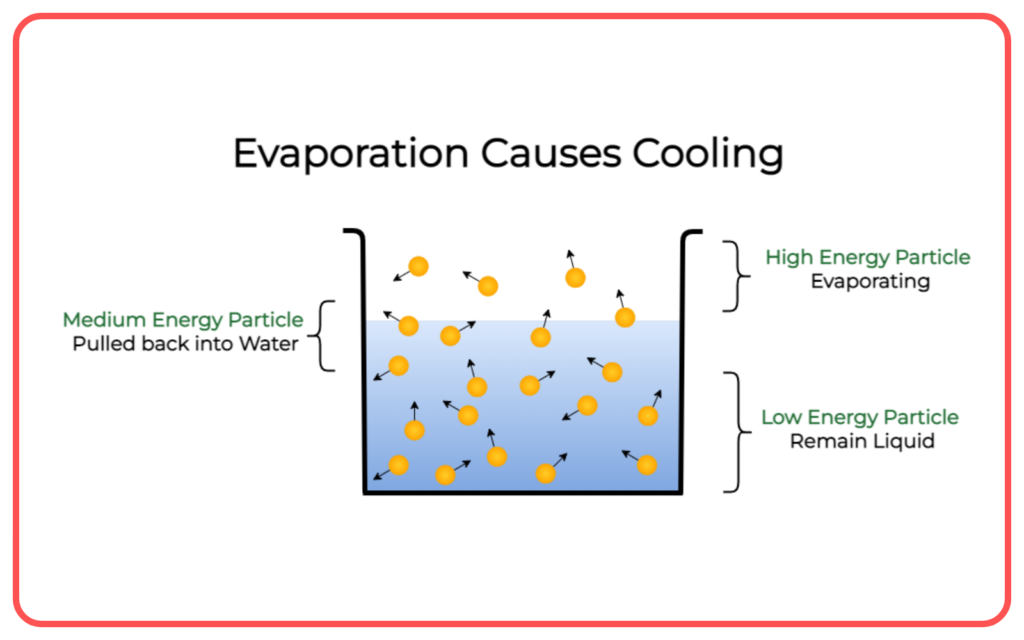
- Molecule Movement: Molecules in a liquid are constantly moving, but those at the surface with high kinetic energy break free into the air.
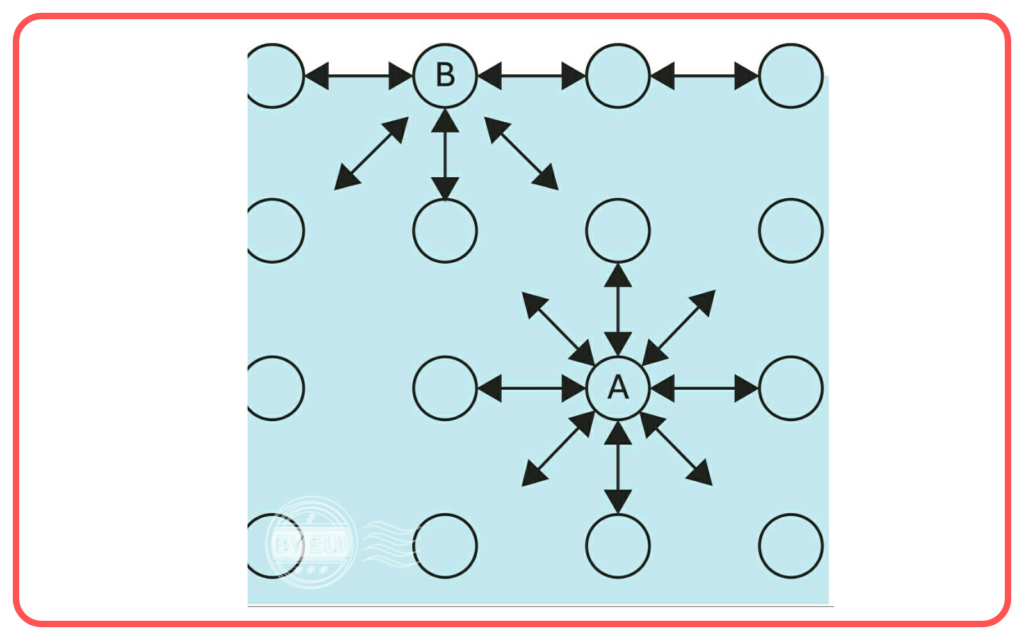
- Cooling Effect: During evaporation, the remaining liquid cools down because the fastest-moving molecules (those with the most energy) escape first.
Factors Affecting Evaporation
Several factors affect the rate of evaporation:
- Temperature: Higher temperatures increase the rate of evaporation because more molecules have enough energy to escape.
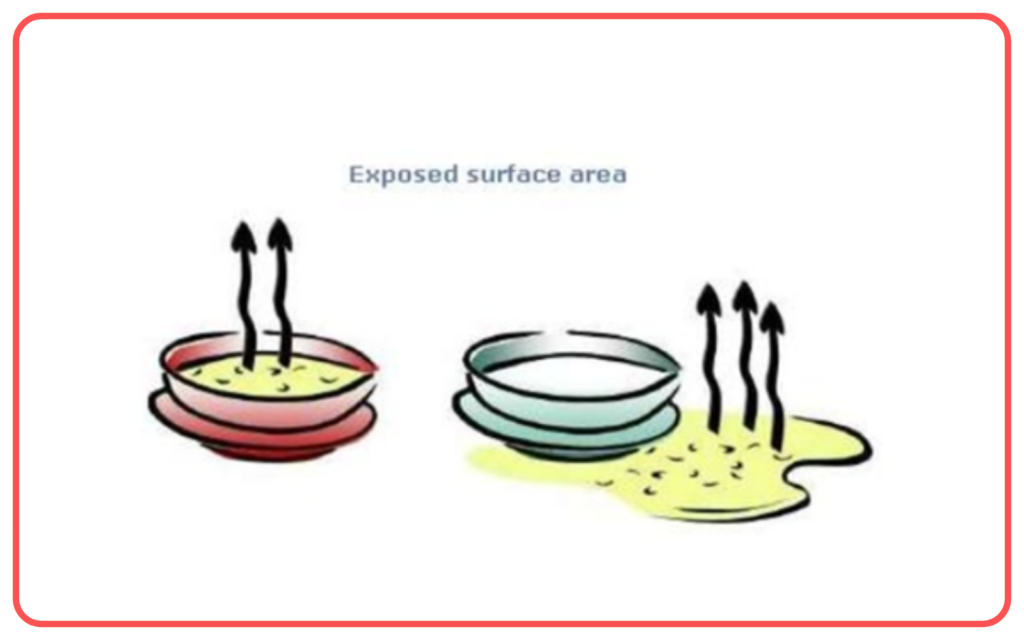
- Surface Area: The larger the surface area, the faster the rate of evaporation because more molecules are exposed to the air.
- Humidity: Evaporation is slower in high humidity because the air is already saturated with water vapor, reducing the ability of additional molecules to escape.

- Wind Speed: Wind or air movement helps in carrying away the vapor from the surface, thus increasing the rate of evaporation.

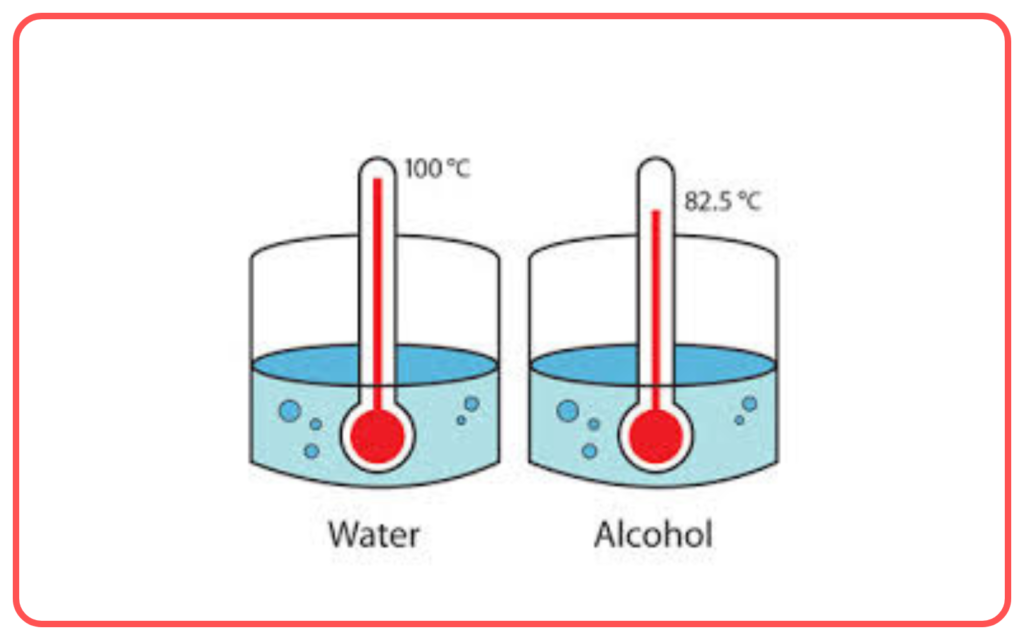
- Nature of the Liquid: Liquids with weak intermolecular forces (e.g., alcohol) evaporate more quickly than those with strong intermolecular forces (e.g., water).
Examples of Evaporation
- Drying of clothes: Clothes dry faster on a sunny, windy day because the water in the clothes evaporates more quickly.

- Water bodies: Evaporation from lakes, rivers, and oceans contributes to the water cycle by forming water vapor in the atmosphere.
- Sweating: The evaporation of sweat from the body helps in cooling the body down.

- Boiling of water: Even before reaching the boiling point, water will begin to evaporate at room temperature.
Applications of Evaporation
- Cooling systems: Evaporative coolers (or swamp coolers) use the process of evaporation to cool air in hot, dry climates.

- Concentration of solutions: Evaporation is used to remove the solvent from a solution, leaving behind the solute (e.g., evaporating water from seawater to get salt).

- Drying of materials: In industries, evaporation is used to dry food, paint, or other products.

- Water cycle: Evaporation is a crucial process in the natural water cycle, where water vapor rises into the atmosphere and eventually condenses to form clouds.
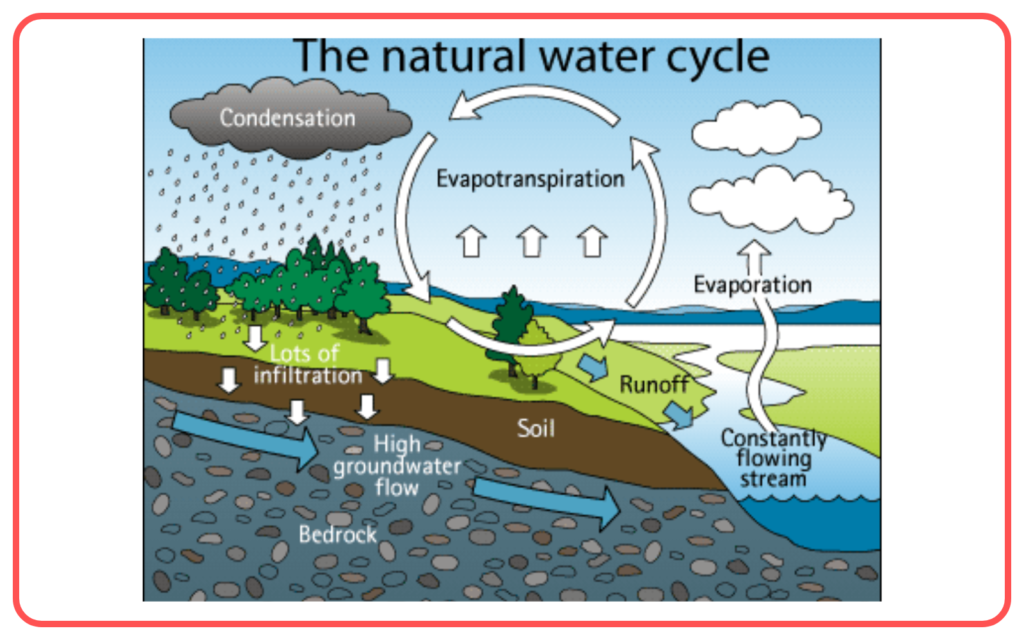
Evaporation vs Boiling
- Evaporation occurs at any temperature and only at the surface of the liquid, while boiling occurs throughout the entire liquid at its boiling point.
- In boiling, bubbles form and rise to the surface, whereas in evaporation, only surface molecules escape.

- Evaporation is a slow process, while boiling is rapid and occurs only at a specific temperature (the boiling point).
The Cooling Effect of Evaporation
- Evaporation has a cooling effect on the remaining liquid. This is because the most energetic molecules (those that are faster-moving) escape the liquid, leaving behind molecules with lower energy.
- This principle is used in sweating (cooling of the body) and in evaporative cooling systems.
- Example: When you are sweating, the body loses heat as the sweat evaporates, helping to maintain a constant body temperature.
Conclusion
- Evaporation is a natural and important process that plays a key role in both nature and technology.
- Understanding the factors that influence evaporation helps in various applications, such as drying, cooling systems, and the water cycle.
Let’s practice!

