Thermal Equilibrium – Heat And Temperature
Key Notes:
Introduction to Heat and Temperature
- Heat is a form of energy that is transferred from a hotter object to a cooler one. It flows due to the difference in temperature between two bodies.
- Temperature is the measure of the average kinetic energy of the particles in an object. It determines how hot or cold an object is.
Difference Between Heat and Temperature
- Heat:
- Measured in joules (J).
- Represents the total energy of molecular motion in a substance.
- Heat flows from a region of high temperature to a region of low temperature.
- Temperature:
- Measured in Celsius (°C), Kelvin (K), or Fahrenheit (°F).
- Represents the average kinetic energy of the particles in a substance.
- Does not depend on the amount of substance but on its thermal state.
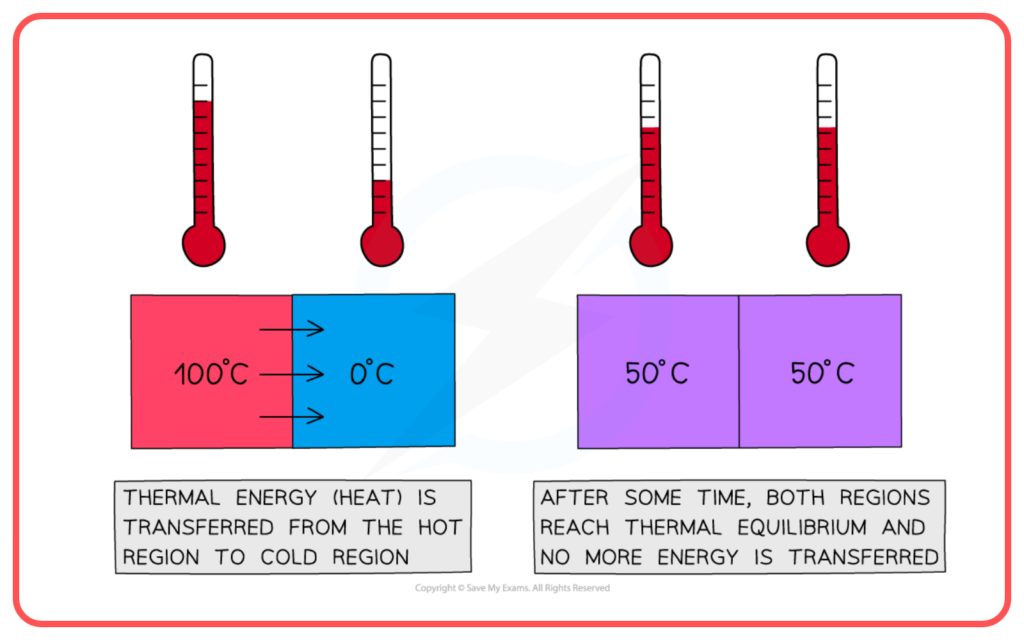
What is Thermal Equilibrium?
- Thermal equilibrium occurs when two bodies are in contact and there is no net flow of heat between them. This means both bodies are at the same temperature.
- When thermal equilibrium is reached, the heat energy transferred between the bodies becomes zero because the temperatures are the same.
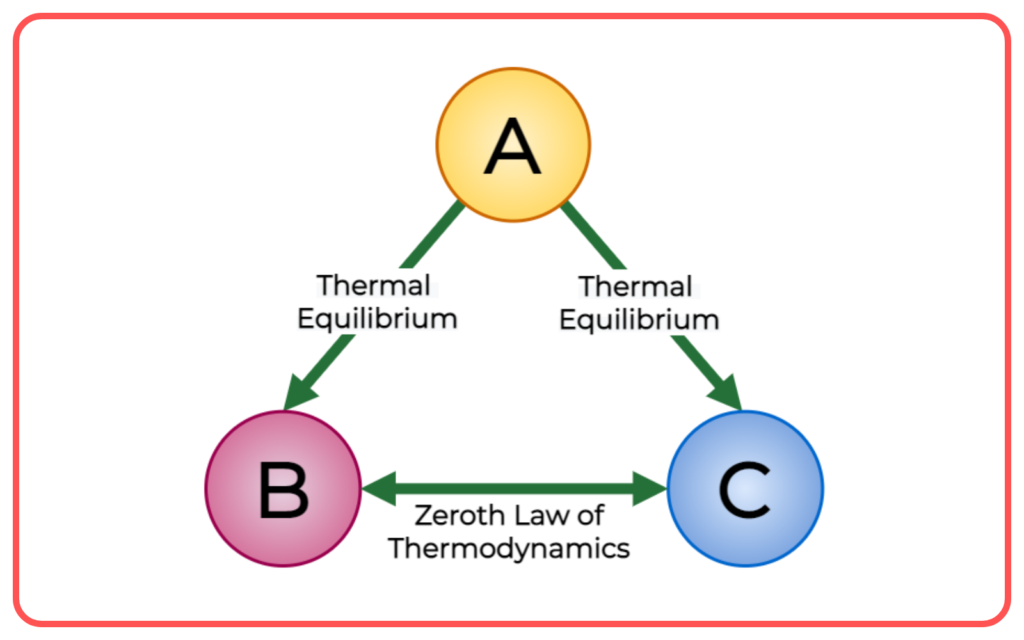
Zeroth Law of Thermodynamics
- The Zeroth Law of Thermodynamics states that:
- If two bodies are each in thermal equilibrium with a third body, then they are in thermal equilibrium with each other.
- This principle allows the definition of temperature. It implies that temperature is a fundamental property that determines whether two bodies are in thermal equilibrium.
- Example: If object A is in thermal equilibrium with object B, and object B is in thermal equilibrium with object C, then object A is in thermal equilibrium with object C.
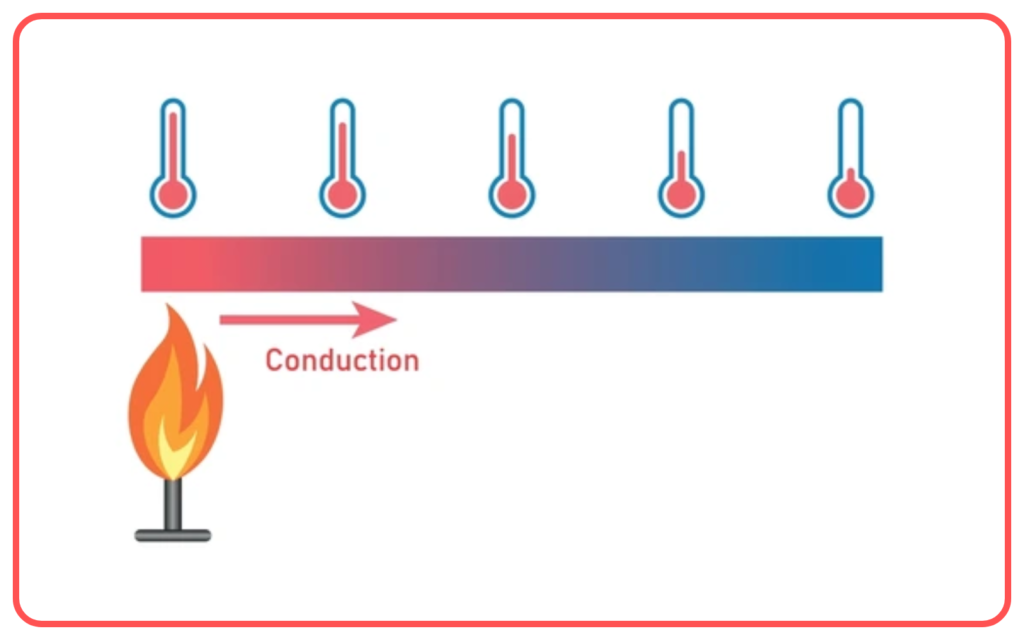
Heat Transfer Methods
- Heat can be transferred in three main ways:
- Conduction:
- Transfer of heat through direct contact between particles in a substance.
- Occurs in solids, where particles vibrate and pass on heat energy.
- Example: Heat flowing through a metal rod.
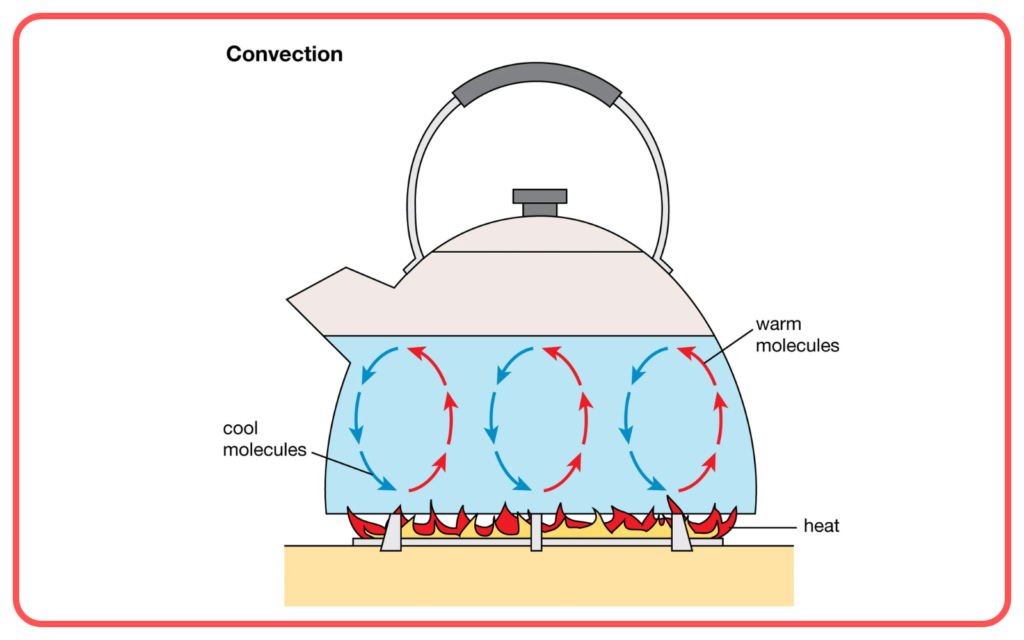
- Convection:
- Transfer of heat through the movement of fluids (liquids and gases).
- Occurs when warmer parts of a fluid rise and cooler parts sink, creating a cycle.
- Example: Warm air rising near a heater, or water heating in a pot.
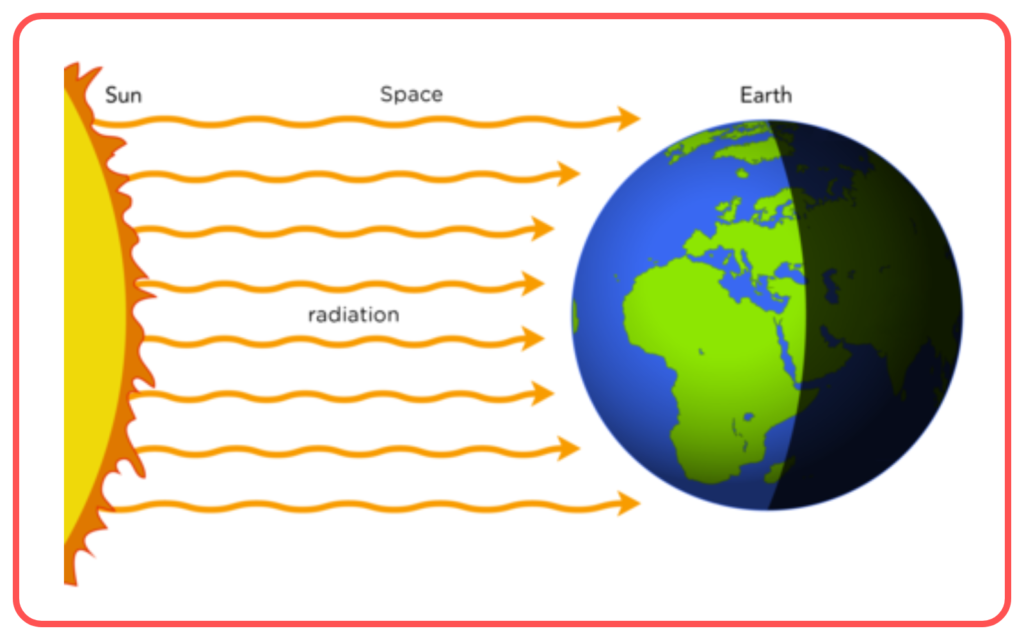
- Radiation:
- Transfer of heat in the form of electromagnetic waves (infrared radiation).
- Does not require a medium (can happen in a vacuum).
- Example: Heat from the Sun reaching Earth.
Temperature Measurement
- Thermometers are used to measure temperature.
- Mercury thermometers: Use mercury that expands and contracts with temperature.
- Digital thermometers: Use electronic sensors to measure temperature.
- Thermometers in everyday life measure the temperature of air, water, and objects.
- Temperature can be measured in different scales:
- Celsius (°C): Freezing point of water is 0°C and boiling point is 100°C.
- Kelvin (K): Used in scientific contexts. The absolute zero is 0 K (-273.15°C).
- Fahrenheit (°F): Used mainly in the United States. Freezing point of water is 32°F, and boiling point is 212°F.
Specific Heat Capacity
- Specific heat capacity is the amount of heat required to raise the temperature of 1 kg of a substance by 1°C (or 1 K).
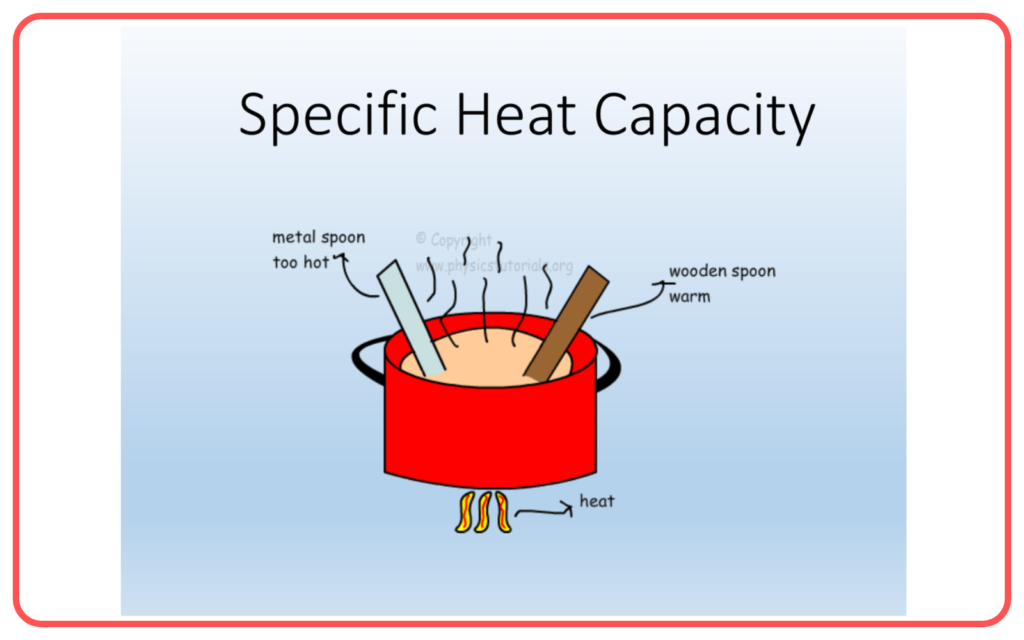
- The formula for specific heat capacity is: Q=mcΔTQ ,where:
- Water has a high specific heat capacity, meaning it can absorb a lot of heat before its temperature rises significantly, making it a good coolant.
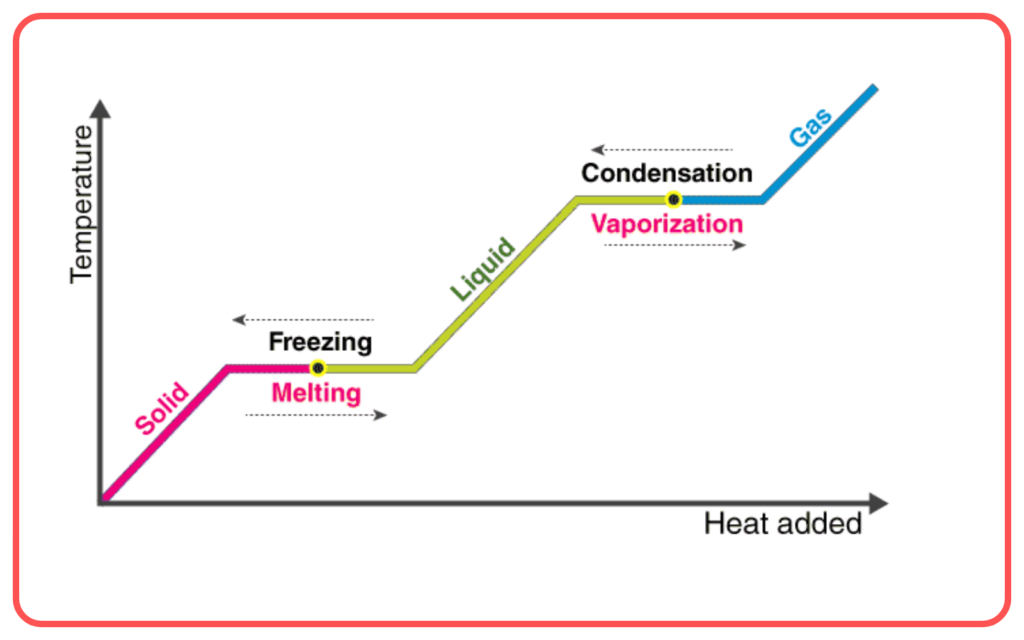
Latent Heat
- Latent heat is the amount of heat required to change the phase of a substance without changing its temperature.
- Latent heat of fusion: Heat required to convert a solid into a liquid (e.g., melting ice).
- Latent heat of vaporization: Heat required to convert a liquid into a gas (e.g., boiling water).
- Formula for Latent Heat: Q=mLQ = mL where:
- Q is the heat energy (in joules).
- m is the mass of the substance (in kg).
- L is the latent heat (in J/kg).
Applications of Heat and Temperature Concepts
- Thermal Insulation:
- Materials like wool, fiberglass, and polystyrene are used to reduce heat transfer and keep buildings warm in winter and cool in summer.

- Engineering:
- Concepts of heat transfer and thermal equilibrium are important in designing engines, refrigerators, and heat exchangers.

- Daily Life:
- Cooking, cooling systems, air conditioning, and the design of electrical devices rely on principles of heat and temperature.

Conclusion
- Heat and temperature are essential concepts in understanding how energy is transferred in different systems.
- Thermal equilibrium is the state in which two objects in contact no longer exchange heat because they have reached the same temperature.
- Understanding how heat is transferred (conduction, convection, and radiation) and how substances respond to heat (specific heat and latent heat) is critical in real-life applications and scientific phenomena.
Let’s practice!

